Nickel-Catalyzed Sila-Cycloaddition of Dichlorodisilanes: Selective Si─Cl Activation for Cyclic Disilanes and Enantioenriched Synthesis
Dr. Liangliang Qi
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Both authors contributed equally to this work.
Search for more papers by this authorShaojie Xu
Department of Chemistry and Materials Science, School of Science, Xi'an Jiaotong-Liverpool University, Suzhou, 215123 China
Both authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Xiaobo Pang
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorYu-Ke Wu
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Xiaotai Wang
Department of Chemistry and Materials Science, School of Science, Xi'an Jiaotong-Liverpool University, Suzhou, 215123 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Xing-Zhong Shu
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Liangliang Qi
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Both authors contributed equally to this work.
Search for more papers by this authorShaojie Xu
Department of Chemistry and Materials Science, School of Science, Xi'an Jiaotong-Liverpool University, Suzhou, 215123 China
Both authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Xiaobo Pang
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorYu-Ke Wu
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Xiaotai Wang
Department of Chemistry and Materials Science, School of Science, Xi'an Jiaotong-Liverpool University, Suzhou, 215123 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Xing-Zhong Shu
State Key Laboratory of Applied Organic Chemistry (SKLAOC) and College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Lanzhou, 730000 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
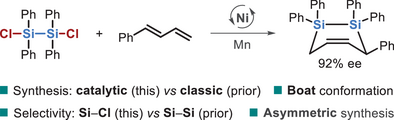
A nickel-catalyzed [4 + 2] sila-cycloaddition between dichlorodisilanes and 1,3-dienes enables efficient access to diverse cyclic disilanes via selective Si─Cl activation. The method tolerates broad diene scope, offers an enantioselective variant, and reveals a unique Ni⁰-mediated SN2-type activation pathway.
Abstract
Disilanyl architectures holds significant value across diverse fields, particularly in the development of advanced materials. However, their limited accessibility has hindered broader exploration. Herein, we report a catalytic strategy for the synthesis of structurally diverse cyclic disilanes through a [4 + 2] sila-cycloaddition between readily available dichlorodisilanes and 1,3-dienes. This transformation proceeds under mild, reductive nickel catalysis via an unusual Si─Cl bond activation pathway and accommodates a broad range of dienes, including bulk feedstocks, cyclic, multi-substituted, aliphatic, aromatic dienes, and trienes. An asymmetric variant further enables the efficient construction of chiral disilane frameworks with high enantioselectivity. The resulting disilacarbocycle adopts boat-like conformations, as revealed by X-ray analysis, due to the elongated Si─Si and Si─C bonds. Mechanistic studies suggest that a triplet Ni0 species engages in an SN2-type oxidative addition to the Si─Cl bond, favoring this pathway over a concerted Si─Si activation. The resulting Si─Ni(I) intermediates are energetically preferred over Si─Ni(II) analogs in the subsequent diene insertion step.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202509961-supp-0001-SuppMat.pdf13.8 MB | Supporting Information |
| anie202509961-supp-0002-SuppMat.zip322 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews: R. Ramesh, D. S. Reddy, J. Med. Chem. 2018, 61, 3779–3798.
- 2Z. Zhou, L. Gai, L.-W. Xu, Z. Guo, H. Lu, Chem. Sci. 14, 10385.
- 3T. A. Su, H. Li, R. S. Klausen, N. T. Kim, M. Neupane, J. L. Leighton, M. L. Steigerwald, L. Venkataraman, C. Nuckolls, Acc. Chem. Res. 2017, 50, 1088.
- 4H. Sakurai, Pure Appl. Chem. 1987, 59, 1637.
- 5V. Pophristic, L. Goodman, C. T. Wu, J. Phys. Chem. A. 2001, 105, 7454–7459.
- 6M. Kumada, K. Tamao, T. Takubo, M. Ishikawa, J. Organomet. Chem. 1967, 9, 43–55.
- 7R. J. P. Corriu, G. F. Lanneau, D. Leclercq, D. Samate, J. Organomet. Chem. 1978, 144, 155–164.
- 8T. J. Barton, J. A. Kilgour, J. Am. Chem. Soc. 1976, 98, 7746–7752.
- 9H. Sakurai, Y. Nakadaira, T. Kobayashi, J. Am. Chem. Soc. 1979, 101, 487–488.
- 10S. reviews: A. K. Franz, K. A. Woerpel, Acc. Chem. Res., 2000, 33, 813–820.
- 11L. Li, Y. Zhang, L. Gao, Z. Song, Tetrahedron Lett. 2015, 56, 1466–1473.
- 12Q.-C. Mu, J. Chen, C.-G. Xia, L.-W. Xu, Coord. Chem. Rev. 2018, 374, 93–113.
- 13T. Hiyama, M. Oestreich, Organosilicon Chemistry: Novel Approach and Reactions; Wiley-VCH: Weinheim, 2019.
10.1002/9783527814787 Google Scholar
- 14Z.-T. Ye, Z.-W. Wu, X.-X. Zhang, J. Zhou, J.-S. Yu, Chem. Soc. Rev. 2024, 53, 8546–8562.
- 15Selected examples of [4+1] reactions: K. Ikenaga, K. Hiramatsu, N. Nasaka, S. Matsumoto, J. Org. Chem. 1993, 58, 5045–5047.
- 16K. Tamao, S. Yamaguchi, M. Shiozaki, Y. Nakagawa, Y. Ito, J. Am. Chem. Soc. 1992, 114, 5867–5869.
- 17T. Ohmura, K. Masuda, I. Takase, M. Suginome, J. Am. Chem. Soc. 2009, 131, 16624–16625.
- 18J. Huo, K. Zhong, Y. Xue, M. Lyu, Y. Ping, Z. Liu, Y. Lan, J. Wang, J. Am. Chem. Soc. 2021, 143, 12968–12973.
- 19W. Lu, Y. Zhao, F. Meng, J. Am. Chem. Soc. 2022, 144, 5233–5240.
- 20L. Qi, Q.-Q. Pan, X.-X. Wei, X. Pang, Z. Liu, X.-Z. Shu, J. Am. Chem. Soc. 2023, 145, 13008–13014.
- 21B. Fu, L. Wang, K. Chen, X. Yuan, J. Yin, S. Wang, D. Shi, B. Zhu, W. Guan, Q. Zhang, T. Xiong, Angew. Chem. Int. Ed. 2024, 63, e202407391.
- 22Selected examples of [4 + 2] reactions: R. Shintani, K. Moriya, T. Hayashi, J. Am. Chem. Soc. 2011, 133, 16440–16443.
- 23W. Wang, S. Zhou, L. Li, Y. He, X. Dong, L. Gao, Q. Wang, Z. Song, J. Am. Chem. Soc. 2021, 143, 11141.
- 24X.-B. Wang, Z.-J. Zheng, J.-L. Xie, X.-W. Gu, Q.-C. Mu, G.-W. Yin, F. Ye, Z. Xu, L.-W. Xu, Angew. Chem. Int. Ed. 2020, 59, 790.
- 25Selected example of [4 + 3] reaction: W.-T. Zhao, F. Gao, D. Zhao, Angew. Chem. Int. Ed. 2018, 57, 6329–6332.
- 26Selected examples of [4 + 4] reactions: K. Hirano, H. Yorimitsu, K. Oshima, Org. Lett. 2008, 10, 2199–2201.
- 27S. Okumura, F. Sun, N. Ishida, M. Murakami, J. Am. Chem. Soc. 2017, 139, 12414–12417.
- 28M.-H. Zhu, X.-W. Zhang, M. Usman, H. Cong, W.-B. Liu, ACS Catal. 2021, 11, 5703–5708.
- 29Selected examples of [3 + 2] reactions: Y. Liang, W. Geng, J. Wei, Z. Xi, Angew. Chem. Int. Ed. 2012, 51, 1934.
- 30M. Onoe, K. Baba, Y. Kim, Y. Kita, M. Tobisu, N. Chatani, J. Am. Chem. Soc. 2012, 134, 19477–19488.
- 31B. Chen, X.-F. Wu, Org. Lett. 2019, 21, 2899–2902.
- 32Selected examples of [2 + 1] reactions: J. Cirakovic, T. G. Driver, K. A. Woerpel, J. Am. Chem. Soc. 2002, 124, 9370–9371.
- 33W. S. Palmer, K. A. Woerpel, Organometallics 1997, 16, 1097–1099.
- 34Selected examples of [5 + 1] reactions: T. He, G. Wang, V. Bonetti, H. F. T. Klare, M. Oestreich, Angew. Chem. Int. Ed. 2020, 59, 12186–12191.
- 35Selected reviews: H. K. Sharma, K. H. Pannell, Chem. Rev. 1995, 95, 1351.
- 36K. A. Horn, Chem. Rev. 1995, 95, 1317–1350.
- 37I. Beletskaya, C. Moberg, Chem. Rev. 2006, 106, 2320–2354.
- 38M. B. Ansell, O. Navarro, J. Spencer, Coord. Chem. Rev. 2017, 336, 54–77.
- 39P. Xiao, L. Gao, Z. Song, Chem.-Eur. J. 2019, 25, 2407.
- 40S. Geng, Y. Pu, S. Wang, Y. Ji, Z. Feng, Chem. Commun. 2024, 60, 3484–3506.
- 41A. Bottoni, A. Perez Higueruelo, G. P. A. Miscione, J. Am. Chem. Soc. 2002, 124, 5506–5513.
- 42J. Zhang, S. Liu, T. Zhang, T. Liu, Y. Lan, Dalton Trans. 2021, 50, 7656–7666.
- 43M. D. Allendorf, C. F. Melius, J. Phys. Chem. 1993, 97, 720–728.
- 44M. Suginome, Y. Ito, Chem. Rev. 2000, 100, 3221–3256.
- 45H. Sakurai, Y. Kamiyama, Y. Nakadaira, Chem. Lett. 4, 1975, 887–890.
10.1246/cl.1975.887 Google Scholar
- 46H. Matsumoto, I. Matsubara, T. Kato, K. Shono, H. Watanabe, Y. Nagai, J. Organomet. Chem. 1980, 199, 43–47.
- 47H. Matsumoto, K. Shono, A. Wada, I. Matsubara, H. Watanabe, Y. Nagai, J. Organomet. Chem. 1980, 199, 185–193.
- 48H. Sakurai, Y. Eriyama, Y. Kamiyama, Y. Nakadaira, J. Organomet. Chem. 1984, 264, 229–237.
- 49An elegant work that highlights the significance and challenges of developing TM-catalyzed C–Si couplings of chlorosilanes, see: B. Vulovic, A. P. Cinderella, D. A. Watson, ACS Catal. 2017, 7, 8113–8117.
- 50For alternative and elegant strategies for chlorosilane activation, see a recent review: Y.-H. Yang, X. Pang, X.-Z. Shu, Synthesis 2023, 55, 868–876.
- 51Titanium catalysis: J. Terao, N. Kambe, Chem. Rec. 2007, 7, 57–67.
- 52Silver catalysis: K. Murakami, K. Hirano, H. Yorimitsu, K. Oshima, Angew. Chem. Int. Ed. 2008, 47, 5833–5835.
- 53Palladium catalysis: Y. Naganawa, K. Sakamoto, Y. Nakajima, Org. Lett. 2021, 23, 601–606.
- 54W. B. Reid, J. R. McAtee, D. A. Watson, Organometallics 2019, 38, 3796–3803.
- 55Chromium catalysis: C. Li, S. Yang, X. Zeng, ACS Catal. 2023, 13, 2062.
- 56Iron catalysis: Y. Lin, L. Zou, R. Bai, X.-Y. Ye, T. Xie, Y. Ye, Org. Chem. Front. 2023, 10, 3052–3060.
- 57Cobalt catalysis: D. Xing, J. Liu, D. Cai, B. Huang, H. Jiang, L. Huang, Nat. Commun. 2024, 15, 4502.
- 58Electrocatalysis: L. Lu, J. C. Siu, Y. Lai, S. Lin, J. Am. Chem. Soc. 2020, 142, 21272–21278.
- 59A. review: X. Pang, X.-Z. Shu, Chem.-Eur. J. 2023, 29, e202203362.
- 60Selected examples: J. Duan, K. Wang, G.-L. Xu, S. Kang, L. Qi, X.-Y. Liu, X.-Z. Shu, Angew. Chem. Int. Ed. 2020, 59, 23083.
- 61L. Zhang, M. Oestreich, Angew. Chem. Int. Ed. 2021, 60, 18587–18590.
- 62H. Cui, C. Niu, M. Xing, C. Zhang, Chem. Commun. 58, 11989–11992, 2022, 58, 11989.
- 63J. Sun, Y. Zhou, R. Gu, X. Li, A. Liu, X. Zhang, Nat. Commun. 2022, 13, 7093.
- 64Z.-Z. Zhao, X. Pang, X.-X. Wei, X.-Y. Liu, X.-Z. Shu, Angew. Chem. 2022, 134, e202200215.
- 65Q.-Q. Pan, L. Qi, X. Pang, X.-Z. Shu, Angew. Chem. Int. Ed. 2023, 62, e202215703.
- 66L.-Y. Yang, Y. Qin, Z. Zhao, D. Zhao, Angew. Chem. Int. Ed. 2024, e202407773.
- 67Selected reviews on reductive nickel and cobalt catalysis: D. J. Weix, Acc. Chem. Res. 2015, 48, 1767–1775.
- 68J. Liu, Y. Ye, J. L. Sessler, H. Gong, Acc. Chem. Res. 2020, 53, 1833–1845.
- 69A. Tortajada, M. Börjesson, R. Martin, Acc. Chem. Res. 2021, 54, 3941–3952.
- 70X. Pang, P.−F. Su, X.−Z. Shu, Acc. Chem. Res. 2022, 55, 2491–2509.
- 71C. A. Herbert, E. R. Jarvo, Acc. Chem. Res. 2023, 56, 3313–3324.
- 72G. A. Dawson, E. H. Spielvogel, T. Diao, Acc. Chem. Res. 2023, 56, 3640–3653.
- 73L.-M. Chen, S. E. Reisman, Acc. Chem. Res. 2024, 57, 751–762.
- 74C. J. Levy, R. J. Puddephatt, J. Am. Chem. Soc. 1997, 119, 10127–10136.
- 75 CCDC 2409201 (4a) contains the supplementary crystallographic data for this paper.
- 76J. F. Chiang, S. H. Bauer, J. Am. Chem. Soc. 1969, 91, 1898–1901.
- 77F. R. Jensen, C. H. Bushweller, J. Am. Chem. Soc. 1969, 91, 5774–5782.
- 78H. Qiu, B. Shuai, Y.-Z. Wang, D. Liu, Y.-G. Chen, P.-S. Gao, H.-X. Ma, S. Chen, T.-S. Mei, J. Am. Chem. Soc. 2020, 142, 9872–9878.
- 79 The reduction potentials of 2a were observed around -2.43 V and -3.06 V vs Ag/Ag+ (see Figure S1 in the SI for details), suggesting that the Ni(II) can be preferentially reduced over chlorosilanes in the presence of reductant.
- 80T. Kerackian, D. Bouyssi, G. Pilet, M. Médebielle, N. Monteiro, J. C. Vantourout, A. Amgoune, ACS Catal. 2022, 12, 12315–12325.
- 81 Please see ref. 23 for related processes on carbon chemistry.