Large N-Doped Zigzag Hydrocarbon Belts from Calixarenes: Synthesis, Structure, and Properties
This is dedicated to Prof. Jieping Zhu on the occasion of his 60th birthday
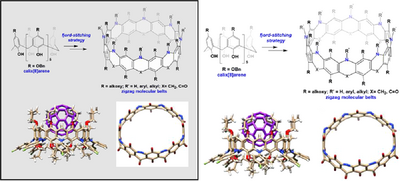
Graphical Abstract
Zigzag molecular nanobelts inlaid with nitrogen atoms and carbonyl groups around portals were synthesized from calix[8]arenes. The synthesis features one-pot multiple intermolecular fjord-stitching C─N bond cross-coupling reactions and selective methylene oxidation reactions. The belt molecules adopt an octagonal prism shape of a large cavity, and are capable of complexing C60 or C70.
Abstract
Zigzag hydrocarbon belts and heteroatom-doped analogs have captivated chemists because of their unique structures, tantalizing properties, and potential applications. Despite synthetic breakthroughs, the diversity, especially, the size of belts synthesized by fjord-stitching approach is limited by resorcin[n]arenes (n = 4, 6). We report herein the synthesis, structure and properties of large belts starting from conventional calix[8]arenes. The synthesis features multiple C─N cross-coupling reactions of per-brominated calix[8]arenes with anilines and ammonia, and selective transformation of 1,4-dihydroquinoline into quinolin-4(1H)-one segments. The molecules in question adopt a unique zigzag belt structure with nitrogen atoms and functional groups located around both portals, yielding a large octagonal prism cavity. They are able to accommodate fullerene C60 or C70 with high affinity driven by lone-pair electron/π interactions and steric complementarity between host and guest.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.