Selection of Ribofuranose-Isomer Among Pentoses by Phosphorylation with Diamidophosphate
In memory of Professor Albert Eschenmoser – on the occasion of his 100 birthday
Graphical Abstract
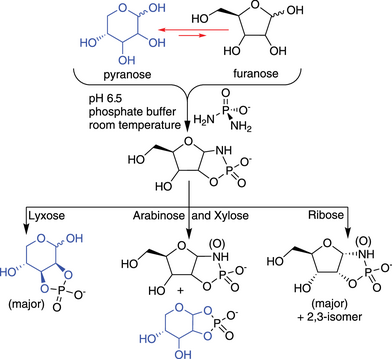
Diamidophosphate phosphorylates the four pentoses under mild aqueous conditions. Ribofuranosyl-cyclicphosphates are formed efficiently, while the other three pentoses react slowly to form furanosyl- and/or pyranosyl-forms. The selective formation of ribofuranosyl-1,2-cyclicphosphate occurs even when starting from a mixture of sugars suggesting an alternative pathway for abiotic synthesis of ribonucleotides in a prebiotic context.
Abstract
The unique structure–function relationship of the ribofuranose ring in RNA has inspired various chemical pathways to select for the ribofuranose form (over the ribopyranose form), especially in a prebiotic context. Herein we show that the reaction of diamidophosphate (DAP) with the four pentoses—ribose, arabinose, xylose, and lyxose—naturally selects the ribofuranose form more efficiently when compared to the other three pentoses. All four pentoses form the initial diamidophosphate-adduct; however, ribose undergoes rapid conversion to ribofuranose-1,2- and 2,3-cyclicphosphate products, while the other three pentoses show significant accumulation of the initially formed DAP-adducts and slow conversion to the cyclicphosphate products. Arabinose and xylose show a distribution between furanose- and pyranose-1,2-cyclicphosphate products, while lyxose forms the furanose-1,2- and pyranose-2,3-cyclicphosphate products. This trend is also manifested when starting from a mixture of pentoses where ribofuranose 1,2-amidocyclicphosphate dominates the product distribution. Such selection for ribofuranose among pentoses by phosphorylation i) provides another venue of how a ribofuranose structure can selectively emerge through abiotic chemical reaction suitable for elaboration toward RNA and ii) adds to the body of experimental work addressing the chemical origin of RNA.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.