Modular Access to P(V)-Stereogenic Compounds via Cu-Catalyzed Desymmetrization of (Thio)Phosphonic Dichlorides
Graphical Abstract
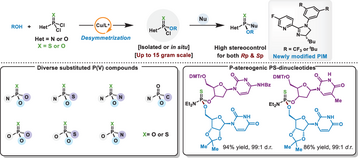
A Cu-catalyzed desymmetrization strategy using newly modified chiral PIM ligands enables modular access to diverse P(V)-stereogenic compounds with high stereocontrol. This approach tolerates broad functionality, constructs challenging P═S motifs, and enables late-stage nucleoside derivatization, providing a versatile platform for drug and nucleic acid-based therapeutic development.
Abstract
Chiral phosphorus(V) centers—particularly those bearing fully heteroatom-substituted frameworks—are key stereochemical motifs in pharmaceuticals, nucleic acid therapeutics, and functional materials. However, their stereoselective construction remains a long-standing challenge in synthetic chemistry. Here, we report a copper-catalyzed desymmetrization strategy enabled by rationally engineered PIM ligands that affords broad and modular access to structurally diverse P(V)-stereogenic compounds. By harnessing the tunable Lewis acidity and well-defined chiral environment of the catalyst—together with the distinct mechanistic features of chiral Lewis acid catalysis—this strategy overcomes the substrate scope limitations that have constrained traditional approaches. The method demonstrates broad functional group tolerance and delivers high levels of stereocontrol across seven distinct substitution patterns, including the efficient construction of chiral P═S motifs previously considered synthetically challenging. Importantly, the approach enables efficient late-stage derivatization of nucleosides and their analogs, thereby providing a general entry point to stereodefined P(V)-containing bioactive molecules. This work not only expands the utility of transition-metal Lewis acid catalysis in phosphorus chemistry, but also establishes a versatile framework for applications in drug discovery and nucleic acid-based therapeutics where precise stereochemical control is essential.
Conflict of Interests
Chinese patent application on this work has been filed by Shanghai Jiao Tong University on Nov. 12, 2024, with M.S. and H.-Q.Y. listed as inventors (application no. 2024116059723). The patent covers the discovery of this new reaction, the scope of both reactants and their applications in the drug development. The remaining authors declare no competing interests.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.