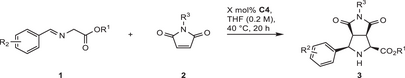
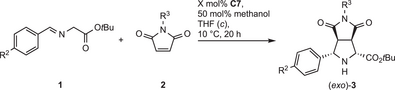
Tunable Endo/Exo Selectivity in Direct Catalytic Asymmetric 1,3-Dipolar Cycloadditions with Polyfunctional Lewis Acid / Azolium–Aryloxide Catalysts
Graphical Abstract
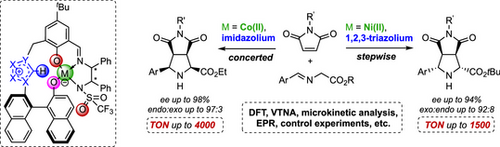
A novel concept for catalytic asymmetric 1,3-dipolar cycloadditions with iminoesters employs modular polyfunctional Lewis acid/azolium-aryloxide betaine catalysts, controlling endo- and exo-selectivity through adjustments of metal center, azolium, and sterics. DFT studies reveal an almost perfect spatial fit of functional sites, enabling precise orchestration of the catalytically relevant moieties involved—reminiscent of enzymatic catalysis.
Abstract
Catalytic asymmetric 1,3-dipolar cycloadditions (1,3-DCA) using iminoesters as ylide precursors offer a powerful approach to accessing stereochemically complex, biologically relevant pyrrolidines. Although previous studies have already achieved impressive stereoselectivities, catalytic productivity remains a challenge, with turnover numbers (TON) typically below 20. In this article, we introduce a novel concept for catalytic 1,3-DCA that enables remarkable productivity for both endo (TON up to 4000) and the more challenging exo products (TON up to 1500). This approach, making use of modular polyfunctional Lewis acid/azolium-aryloxide catalysts, allows for precise control over endo- and exo-diastereoselectivity. The switch from endo- to exo-selectivity is accomplished by modifying the metal center, the azolium moiety, and steric factors. As detailed DFT studies reveal, both the endo- and exo-selective catalyst systems exhibit an almost perfect spatial alignment of their key functional sites, allowing for a unique interplay of Brønsted acids and bases, Lewis acids, and hydrogen bonding. The computational studies further demonstrate that these polyfunctional catalysts dramatically lower the energetic barriers of the concerted or stepwise cycloaddition key steps. However, they also precisely orchestrate and accelerate all accompanying transformations—reminiscent of enzymatic machineries.
Introduction
1,3-Dipolar cycloadditions provide a powerful strategy for the construction of stereochemically complex, highly functionalized 5-membered rings containing heteroatoms.[1, 2] Pyrrolidines are among the most important nitrogen-containing heterocycles. They are found in a huge number of bioactive natural products[3-7] and are essential as chiral key motifs in a large number of active pharmaceutical ingredients,[8] which are prescribed, e.g., as antibacterial, antifungal, and tumor-therapeutic agents.[9-13] This explains the demand for efficient stereoselective methods for the preparation of pyrrolidines. A large number of catalytic asymmetric 1,3-DCA of azomethine ylides and electron-poor olefins has been developed to provide a straightforward, elegant access. Various Lewis acid catalysts have been reported to allow for high stereocontrol despite the fact that up to 16 stereoisomers might, in principle, be formed.[9-13] Silver[19-21] and copper[22-25] catalysts, in particular Carretero's Cu(I) Fesulphos, are well-established and frequently used (Scheme 1, top).[14-18] Other central metals have been occasionally reported.[26-33]
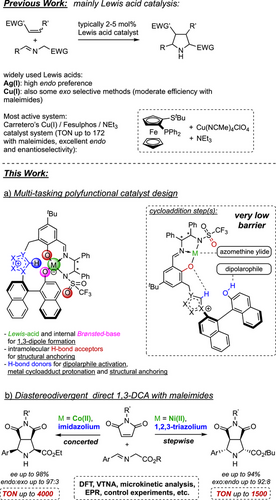
As pointed out in a recent review by Adrio and Carretero, most of the best 1,3-DCA methods using a specific type of dipolarophile manage the efficient synthesis of either endo or exo isomers, whereas stereodivergent approaches providing different diastereomers on demand are quite rare.[14, 34-37]
Using maleimides as dipolarophile, reported catalyst loadings are typically 2–10 mol% to form almost exclusively endo products.[28, 29, 31, 38-53] Carretero's Cu(I)/Fesulphos constitutes an important exception (Scheme 1, top), as catalyst loadings of 0.5 mol% were proficient to allow for TON up to 172.[24] Synthesizing exo diastereomers with a combination of high yields, TONs, enantio- and diastereoselectivity is still a challenge.
- – the metal center: Co(II) versus Ni(II)
- – the azolium unit: imidazolium versus 1,2,3-triazolium
- – the azomethine ylide's ester unit
Results and Discussion
Development and Optimization Studies
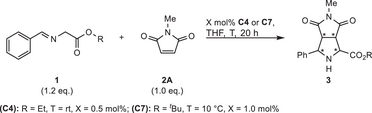
The investigation started with our previously reported Cu(II)/imidazolium-naphthoxide catalyst C1 (Table 1),[54-58] using the 1,3-DCA of glycine iminoethylester 1a with N-methylmaleimide 2A as a model reaction. With 5 mol% catalyst, pyrrolidine 3aA was formed in nearly quantitative yield and with good enantioselectivity, yet low endo-selectivity (entry 1). With the new Zn(II), Ni(II), and Co(II) complexes C2–C4, which were readily prepared in few steps with high yields in close analogy to C1 (see Supporting Information), yields were again very high and stereoselectivity was significantly improved (entries 2–4). Co(II) complex C4 provided the highest diastereoselectivity and was selected for further studies.[59] Its diastereomer C5 showed a mismatched behavior (entry 5), favoring the other enantiomer. The loading of C4 could be largely decreased, still allowing for high stereoselectivity (entries 6–7). With just 0.025 mol% of C4 at 40 °C, 3aA was formed in quantitative yield (TON 4000), while high stereoselectivity was still accomplished (entry 7).
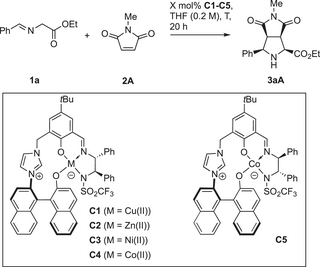 |
|||||||||
| C | M | X (mol%) | T / (°C) | Conv.a) / (%) |
Yielda) / (%) |
endo: exoa) |
eeb) (%) |
TON | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C1 | Cu(II) | 5 | 25 | 99 | 99 | 77:23 | 90 | 20 |
| 2 | C2 | Zn(II) | 5 | 25 | >99 | >99 | 92:8 | 96 | 20 |
| 3 | C3 | Ni(II) | 5 | 25 | >99 | 97 | 91:9 | 99 | 19 |
| 4 | C4 | Co(II) | 5 | 25 | >99 | 99 | 94:6 | 95 | 20 |
| 5 | C5 | Co(II) | 5 | 25 | >99 | >99 | 78:22 | −85 | 20 |
| 6 | C4 | Co(II) | 0.1 | 25 | 99 | 99 | 94:6 | 98 | 990 |
| 7 | C4 | Co(II) | 0.025 | 40 | >99 | >99 | 93:7 | 97 | 4000 |
- a) Determined by 1H-NMR in the presence of an internal standard.
- b) Enantiomeric excess of the endo isomer determined by HPLC. A negative value indicates the major formation of the optical antipode of the one depicted.
During the catalyst screening, different alkoxy residues in the iminoesters were tested. It was found that Ni(II) catalyst C3, which allowed for good endo selectivity with ethylester substrate 1a (Table 1, entry 3), permitted a low exo-preference with tert.-butyl ester 1b (Table 2, entry 1).
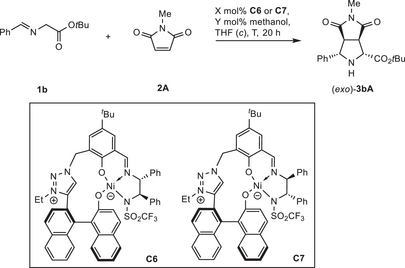 |
||||||||||
| C | X (mol%) | Y (mol%) | c / (M) | T / (°C) | Conv.a)/(%) |
Yielda) /(%) |
endo: exoa) |
ee (%)b) |
TON | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C3 | 5 | − | 0.2 | 25 | 95 | 86 | 30:70 | −66 | 17 |
| 2 | C6 | 5 | − | 0.2 | 25 | 98 | 88 | 11:89 | −72 | 18 |
| 3 | C7 | 5 | − | 0.2 | 25 | 94 | 74 | 20:80 | 90 | 15 |
| 4 | C7 | 5 | − | 0.5 | 0 | 93 | 86 | 11:89 | 93 | 17 |
| 5 | C7 | 5 | − | 1.0 | 0 | 82 | 80 | 8:92 | 93 | 16 |
| 6 | C7 | 0.05 | − | 2.0 | 10 | 85 | 75 | 13:87 | 91 | 1500 |
| 7 | C7 | 0.05 | 50 | 2.0 | 10 | 82 | 75 | 11:89 | 92 | 1500 |
- a) Determined by 1H-NMR in the presence of an internal standard.
- b) Enantiomeric excess of the exo isomer determined by HPLC. Negative values indicate the major formation of the optical antipode of the one depicted.
With 1,2,3-triazolium catalysts C6 and C7 the exo selectivity and enantioselectivity were further improved.[60-65] C6 gave higher exo selectivity, but a moderate ee (entry 2), while its diastereomer C7 allowed for high ee, but moderate exo preference (entry 3).
However, the highest exo- and enantioselectivity could be obtained with C7 at 0 °C at higher concentration (exo:endo = 92:8, ee = 93%, entry 5). Gratifyingly, also for the challenging exo-isomer synthesis, low catalyst loadings can be applied. A TON of 1500 was attained with 0.05 mol% catalyst at 10 °C (entry 6). Upon addition of 50 mol% of methanol, the stereoselectivity could be slightly improved (exo:endo = 89:11, ee = 92%, entry 7).[66]
Reaction Scope
The generality of both the endo- and exo-selective approaches was studied using different iminoester and maleimide derivatives (0.2 mmol). With Co(II) catalyst C4, the reaction conditions of Table 1, entry 7 were used, but adapting catalyst loadings to substrate reactivities. Different loadings were investigated, and the in our eyes most attractive compromise of loading and reaction outcome is listed in Table 3. Methyl, ethyl, and benzyl esters were compared and provided almost identical results (entries 1–3). Apparently, the electronic influence of different substituents R2 on arylimino moiety is relatively small, as all investigated σ- and π-donors and σ- and π-acceptors in the para-position were well accommodated (entries 4, 5, 6, and 9, respectively). Substitution at meta- and ortho-position was studied for a chloro substituent and led to similar results as para-substitution (entries 7–8).[67, 68]
 |
||||||||||
| 3 | 1 | R1 | R2 | 2 | R3 |
X / (mol%) |
Yielda) / (%) |
endo :exob) |
eec) (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | aA | a | Et | H | A | Me | 0.05 | 84 | 93:7 | 97 |
| 1‘d) | aA | a | Et | H | A | Me | 0.1 | 76 | 93:7 | 98 |
| 2 | cA | c | Me | H | A | Me | 0.05 | 83 | 93:7 | 94 |
| 3 | dA | d | Bn | H | A | Me | 0.05 | 82 | 95:5 | 95 |
| 4 | eA | e | Et | 4-OMe | A | Me | 0.1 | 83 | 94:6 | 94 |
| 5 | fA | f | Et | 4-Me | A | Me | 0.1 | 99 | 93:7 | 96 |
| 6 | gA | g | Et | 4-Cl | A | Me | 0.025 | 98 | 92:8 | 93 |
| 7 | hA | h | Et | 3-Cl | A | Me | 0.05 | >99 | 92:8 | 90 |
| 8 | iA | i | Et | 2-Cl | A | Me | 0.2 | >99 | 97:3 | 93 |
| 9 | jA | j | Et | 4-NO2 | A | Me | 0.1 | 97 | 88:12 | 75 |
| 10 | aB | a | Et | H | B | H | 0.3 | 98 | 94:6 | 94 |
| 11 | aC | a | Et | H | C | Ph | 0.025 | 86 | 93:7 | 86 |
| 12 | aD | a | Et | H | D | Bn | 0.1 | 97 | 92:8 | 95 |
- a) Yield of isolated product after column chromatography.
- b) Determined by 1H-NMR from the crude product.
- c) Enantiomeric excess of the endo isomer determined by HPLC.
- d) Scale up to 2.0 mmol.
A notable feature is the tolerance of unprotected maleimide substrate 2B (R3 = H), still providing high yield and stereoselectivity with a C4 loading of 0.3 mol%. With an N-Ph residue R3, very high productivity was found, albeit the ee was decreased to 86%, whereas with R3 = benzyl as a removable protective group, a high ee value was accomplished.
With Ni(II) catalyst C7, the reaction conditions of Table 2, entry 7 were employed, using 0.2–1 mol% as catalyst loading (Table 4). The dependence on substituent R2 (entries 2–5) does not apparently follow an electronic tendency regarding productivity. In all cases, good to high yields (75%–96%) and ee values (87%–94%) were attained. N-Ph and N-Bn were also well tolerated (entries 6 and 7), whereas the unprotected maleimide 2B was unreactive (not shown). In general, the exo-isomer was formed as the major product with moderate (entries 2 and 4) to good (entries 1, 3, 6, and 7) dr values, whereas with R2 = NO2, the dr was low (entry 5).
 |
| (exo)-3 | 1 | R2 | 2 | R3 |
X / (mol%) |
Yielda) / (%) | endo:exob) |
eec) (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| 1 | bA | b | H | A | Me | 0.5 | 95 | 11:89 | 94 |
| 1‘ | bA | b | H | A | Me | 0.2 | 87 | 13:87 | 93 |
| 2 | kA | k | OMe | A | Me | 2.0 | 93 | 20:80 | 90 |
| 3 | lA | l | Cl | A | Me | 0.5 | 96 | 8:92 | 91 |
| 4 | mA | m | Me | A | Me | 0.5 | 92 | 18:82 | 89 |
| 5 | nA | n | NO2 | A | Me | 1.0 | 75 | 31:69 | 87 |
| 6 | bC | b | H | C | Ph | 1.0 | 87 | 10:90 | 91 |
| 7 | bD | b | H | D | Bn | 1.0 | >99 | 9:91 | 91 |
- a) Yield of isolated product after column chromatography.
- b) Determined by 1H-NMR from the crude product.
- c) Enantiomeric excess of the exo isomer determined by HPLC.
- d) Scale up: 3.0 mmol 2A.
Catalyst Recycling
For recyclability studies, catalysts C4 and C7 were separated after 20 h from the reaction mixtures by filtration over silica gel. Silica protonates the active catalysts to give the precatalysts, which stick on silica. After elution of product, active catalysts are regenerated by 1 vol% iPr2NEt (C4) or Et3N in THF (C7). For C4, it was found that the very high yields and stereoselectivities were maintained for at least four cycles (Table 5, entries 1–4). For C7, yields decreased from 91% to 77% after 4 runs, but high stereoselectivity was maintained (entries 5–8).
 |
||||
| Run | C |
Yielda) / (%) |
endo :exob) |
ee Major diastereomerc) (%) |
|---|---|---|---|---|
| 1 | C4 | >99 | 93:7 | 97 |
| 2 | C4 | >99 | 93:7 | 96 |
| 3 | C4 | >99 | 93:7 | 96 |
| 4 | C4 | >99 | 93:7 | 96 |
| 5 | C7 | 91 | 10:90 | 94 |
| 6 | C7 | 87 | 10:90 | 93 |
| 7 | C7 | 89 | 13:87 | 93 |
| 8 | C7 | 77 | 9:91 | 93 |
- a) Yield of isolated product after column chromatography.
- b) Determined by 1H-NMR from the crude product.
- c) Enantiomeric excess of the endo isomer determined by HPLC.
Mechanistic Investigations
Control Experiments
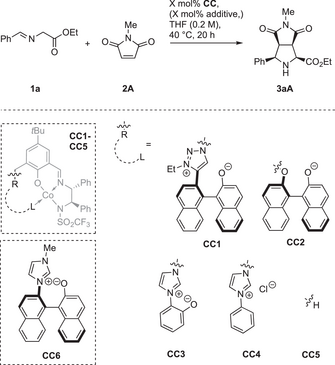
To learn which architecture and functional groups in the polyfunctional systems are essential for their performance, a number of control catalyst systems CC was investigated. For high endo selectivity the imidazolium moiety in C4 is advantageous compared to a 1,2,3-triazolium moiety in the control catalyst CC1 (Table 6, entry 1). Azolium rings are beneficial for high enantioselectivity, as judged from CC2 missing this feature, which gives nearly racemic product (entry 2). Furthermore, the axially chiral betaine element provides activity advantages compared to the simpler imidazolium phenolate in CC3. While good results were attained with 5 mol% of CC3 (entry 3), no product was detected with 0.1 mol% (entry 4). This is in strong contrast to the use of C4 (see Table 1, entries 6–7). Like expected, imidazolium catalyst CC4 lacking the basic aryloxide showed a further decrease in activity (Table 6, entries 5 and 6).[69-75]
 |
|||||||
| X / (mol%) | CC | additive |
conv.a) / (%) |
yielda) / (%) |
endo: exoa) |
eeb) / (%) |
|
|---|---|---|---|---|---|---|---|
| 1 | 5 | CC1 | − | 99 | 89 | 84:16 | 92 |
| 2 | 5 | CC2 | − | 87 | 77 | 89:11 | 14 |
| 3 | 5 | CC3 | − | 90 | 90 | 97:3 | 80 |
| 4 | 0.1 | CC3 | − | <2 | <2 | − | − |
| 5 | 5 | CC4 | − | 63 | 57 | 95:5 | 84 |
| 6 | 0.1 | CC4 | − | <2 | <2 | − | − |
| 7 | 5 | CC5 | − | 99 | 54 | 85:15 | 1 |
| 8 | 5 | CC5 | Cs2CO3 | >99 | 11 | 76:24 | 74 |
| 9 | 5 | CC5 + CC6 | − | >99 | 71 | 67:33 | 82 |
- a) Determined by 1H-NMR in the presence of an internal standard.
- b) Enantiomeric excess of the endo isomer determined by HPLC.
The simple complex CC5 also displayed catalytic activity at high loadings, but gave nearly racemic product (entry 7). In the presence of Cs2CO3 as an external base the formation of large side product quantities was found, resulting in a poor yield, while enantioselectivity was largely improved (entry 8). Combining CC5 with betaine fragment CC6 in a binary catalyst system resulted in decreased endo selectivity and also considerable side product formation. These results indicate that an intramolecular interplay of the various functional groups is essential for the high performance of C4.
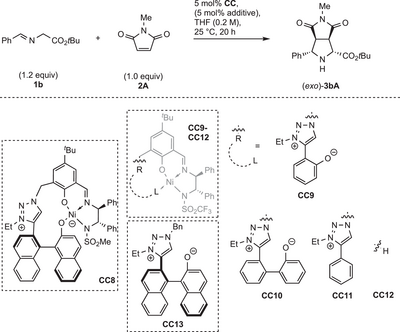
A similar program was performed for the exo-selective reaction (Table 7). Surprisingly, catalyst CC8 featuring an N-mesyl ligand donor provided mainly endo product (entry 1), revealing the impact of the N-triflyl donor in C7. We also investigated phenolate CC9 (entry 2), lacking the element of axial chirality. Compared to the standard system C7, it is less active and does not favor the formation of the exo isomer. The binaphthyl architecture thus seems to help to accomplish a suitable structure to overwrite the inherent endo preference. Also, the aryloxide metal distance seems to be crucial, because for biphenolate CC10, which is structurally more similar to C7, we noticed exo-selectivity (entry 3), yet still much lower than for C7.
 |
||||||
| CC | Additive |
Conv.a) / (%) |
Yielda) / (%) |
exo: endoa) |
ee exob) / (%) |
|
|---|---|---|---|---|---|---|
| 1 | CC8 | − | 97 | 97 | 31:69 | 82 |
| 2 | CC9 | − | 86 | 76 | 46:54 | 83 |
| 3 | CC10 | − | 99 | 98 | 77:23 | 78 |
| 4 | CC11 | − | 0 | 0 | − | − |
| 5 | CC12 | − | 0 | 0 | − | − |
| 6 | CC12 | Cs2CO3 | 0 | 0 | − | − |
| 7 | CC12 + CC13 | − | 36 | 11 | 22:78 | 11 |
- a) Determined by 1H-NMR in the presence of an internal standard.
- b) Enantiomeric excesses determined by HPLC.
Interestingly, the catalysts CC11 and CC12, lacking an aryloxide, showed no catalytic activity (entries 4–6). In contrast, the binary catalyst system of CC12 and CC13 gave a small amount of product (entry 7) with poor stereoselectivity.
Table 7 thus suggests that for the exo-selective synthesis, the sophisticated intramolecular interplay between the Ni center and the triazolium aryloxide with a precisely elaborated chiral environment is crucial for high performance.
Spectroscopic Studies
The properties of C4 and C7 were studied by paramagnetic Evans-NMR, SQUID (superconducting quantum interference device) magnetometry, and EPR to gain insight into the electronic structure of the catalyst in a solid state as well as in solution. SQUID measurements of the molar magnetic susceptibility χm of both C4 and its precatalyst (featuring the neutral naphthol unit) were performed on powder samples. Both samples show a constant χmT of around 2.3 cm3 mol−1 K at high temperatures and a strong decrease below 100 K (Figures S25 and S26).
Simulations of the susceptibility-temperature product χmT for both compounds using the spin Hamiltonian formalism reveals a high-spin state (S = 3/2) with zero-field splitting (ZFS) parameters of D = +38 cm−1 and E/D = 0.30(3).[76] The slight difference in χmT at room temperature between C4 and its precatalyst is due to different giso in C4 (giso = 2.22(1)) and its precatalyst (giso = 2.28(1)). Furthermore, Evans method NMR measurements on C4 and the precatalyst were performed in THF.[77, 78] The paramagnetic shift confirms the high-spin state (S = 3/2), but giso is slightly lower. EPR experiments using frozen solutions confirm these trends and are shown in the Supporting Information (Figure S28).
SQUID measurements on C7 and its precatalyst (again featuring the neutral naphthol unit) show low χmT of 0.07 cm3 mol−1 K across the whole temperature range with a slight increase above 270 K to 0.2 cm3 mol−1 K at 300 K for C7. For a Ni d8-system in a high spin configuration (S = 1) χmT = 1 cm3 mol−1 K (assuming giso = 2) and for low spin (S = 0) χmT = 0 cm3 mol−1 K would be expected. These results indicate that C7 does not exist in a single magnetic conformation and a small part of the compound is paramagnetic. CASSCF calculated magnetic parameters based on PBEh-3c/def2-mSVP geometries support these results. The quantum chemical calculations strongly suggest this species to be the catalytically relevant one.
Concentration-dependent UV–Vis studies of catalysts C4 and C7 show a linear absorption to concentration relationship thus pointing to monomeric catalyst species (see Supporting Information, Figures S32 and S34). This assumption is further supported by DFT computations, according to which the dimerization of the catalysts is either endergonic (C7) or not possible due to steric hindrance (C4). Moreover, the linear dependencies of catalyst ee and product ee values for both systems are consistent with monomeric catalyst species (see Supporting Information, Figures S29 and S30).
Computational and Kinetic Studies
Based on the control experiments and the spectroscopic results in combination with previous experimental and computational studies on related Cu(II) catalysts reported by our groups,[54, 65, 69] we used the simplified general catalytic cycle shown in Scheme 2 as working hypothesis for both the endo- and exo-selective methods.
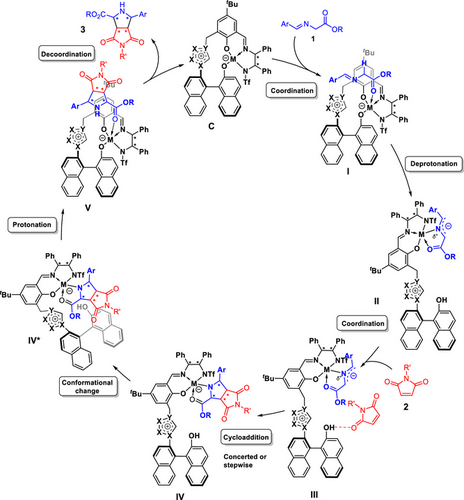
According to this mechanistic model, iminoester 1 initially coordinates to catalyst C, thus forming I. This is followed by a proton shift from the iminoester to the catalyst's naphthoxide moiety, giving azomethine ylide complex II. As a next step, maleimide 2 binds to II via a hydrogen bond to the generated naphthol-OH, forming III. This allows for a quasi-intramolecular (concerted or stepwise) cycloaddition. After the formation of the cycloadduct complex IV, a conformational change of the catalyst to IV* is necessary to enable protonation of the cycloadduct-metal bond by the naphthol moiety to form the catalyst/product adduct V. Subsequent product dissociation closes the catalytic cycle.
Following that mechanistic model, comprehensive computational studies were conducted on the (B3LYP-D3(BJ)/def2-TZVP/ COSMO(THF)) level of theory on PBEh-3c/def2-mSVP geometries (for details see Supporting Information)[79-92] to give—in combination with kinetic and spectroscopic investigations—detailed mechanistic pictures for both the endo- and exo-selective methods. In fact, according to our calculations, both reactions follow the general mechanism outlined above, but differ in various aspects that are important for the catalytic outcome.
Endo-selective Approach With Catalyst C4
As the magnetic measurements in solid state and solution (vide supra) show C4 to be a high-spin Co(II) complex, we evaluated the reaction pathway by DFT calculations on the respective quartet potential energy surface (PES). The predicted Gibbs free energy profile for the endo-configured main stereoisomer with a thermodynamic driving force of ΔrG° = −50.0 kJ mol−1 is shown in Figure 1. Our DFT calculations suggest that the initial iminoester coordination to C4 proceeds in a monodentate way via the ester moiety and is slightly endergonic. This step is followed by a slow and endergonic intramolecular deprotonation of the coordinated iminoester by the naphthoxide moiety to form the ylide-like IICo, in which the Co(II) center adopts a trigonal-bipyramidal geometry with elongated axial bonds. The formally neutral donor atoms coordinate axially, while the negatively charged ones form the equatorial plane. The subsequent imide association via H-bond to the naphthol unit in IIICo is again endergonic.
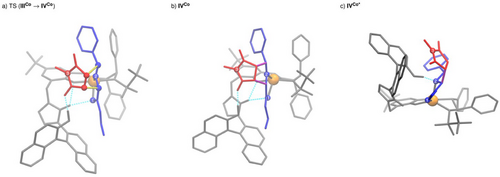
In contrast, the actual cycloaddition step forming two new C,C-bonds is calculated to be strongly exergonic. Interestingly, it proceeds in a concerted manner for the endo pathway, while typically stepwise mechanisms have been suggested and calculated for other catalysts.[93-95] In transition state TS(IIICo→IVCo) (Figure 2, left), in addition to the hydrogen bond activation of the maleimide, the C–H acidic imidazolium forms a double hydrogen bond with the 1,3-dipole and the phenoxy moiety of the ligand. The latter can serve as a structural anchor, rigidifying and stabilizing the assembly. As a result of the simultaneous activation of both substrates adopting a suitable spatial orientation for a quasi-intramolecular step, a remarkably low barrier of 15.5 kJ mol−1 (enthalpic part: 9.8 kJ mol−1, entropic part: 5.7 kJ mol−1) is found for this event thus demonstrating the power of the polyfunctional catalysis concept in this key step. The chiral metal sphere and the axially-chiral binaphthyl moiety form a well-defined chiral binding pocket that stabilizes the spatial orientation required for the concerted mechanisms and effectively reduces the activation entropy. Moreover, by the precisely defined alignment of both substrates within the catalyst's chiral environment, high enantioselectivity is achieved.
For the competing exo-pathway with C4, a stepwise cycloaddition mechanism was found requiring to surpass a significantly higher barrier (see Supporting Information, Figure S37).
The reaction steps following the C,C-bond formations are particularly characteristic for the catalytic mechanism with C4. As shown in Figure 2, the naphthol unit in IVCo forms a hydrogen bond to the phenoxy unit of the ligand sphere. To enable the highly exergonic protonation of the cycloadduct, this bond must be broken, allowing for an isoenergetic conformational change of the imidazolium/naphthol unit. This change of IVCo to its conformer IVCo* restores the trigonal-bipyramidal coordination of the Co(II) center and permits the formation of a hydrogen bond between the naphthol unit and the pyrrolidinate nitrogen of the initial cycloadduct, thus facilitating a quasi-barrierless proton transfer to form VCo. This Brønsted acid/base reaction step closely resembles enzymatically catalyzed proton transfer reactions.[96, 97]
The product release from VCo closes the catalytic cycle by regenerating C4. VCo is energetically favored compared to the iminoester/ catalyst complex ICo by −7.5 kJ mol−1.
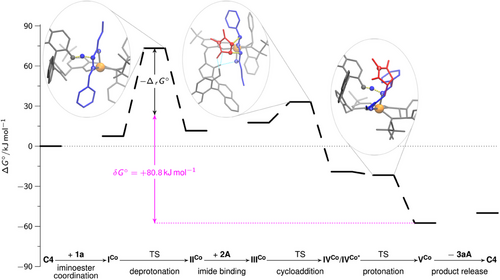
According to the energetic span model,[98] the catalytic activity of a system, measured as TOF, is usually determined by only two states: the turnover-determining intermediate (TDI) and the turnover-determining transition state (TDTS). Whenever the TDI appears before the TDTS in the catalysis cycle, the energetic span is determined by the Gibbs free energy difference between those two states. Otherwise, the energetic span is the sum of that Gibbs free energy difference and the thermodynamic driving force. The energetic span model enables the direct comparison between Gibbs free energy profiles obtained by quantum chemistry and the experimental kinetic investigations. Following the DFT evaluation of our mechanistic model (Figure 1), the energetic span (+80.8 kJ mol−1) of the investigated, endo-selective catalytic reaction is given by VCo as TDI and the TS of the deprotonation step (ICo → IICo,a) as TDTS. Interestingly, the calculated entropic part of the activation barrier is almost zero (0.04 kJ mol−1), which is another similarity to enzymatic catalysis and might be explained by the rigidified catalyst structure, resulting from structural anchoring by H-bond interactions.[99, 100]
Using a microkinetic model, barriers can be assigned to individual chemical steps from experimentally measured concentrations. For this purpose, the ordinary differential equation system resulting from the mechanistic hypothesis is solved, and the barriers of the individual reaction steps are optimized in order to best replicate the experimentally measured concentration profiles. In order to better understand the endo-selective DCA, we decided to fit such a microkinetic model using methods (see Supporting Information) similar to those previously reported for an asymmetric hydroboration by our groups.[102] According to our microkinetic model, the barrier of the iminoester deprotonation has the major influence on the kinetics, which is consistent with the identification of the corresponding TS as TDTS. Furthermore, the apparent barrier for the catalytic reaction 1a + 2A → 3aA from our microkinetic model is found to be +75.6 kJ mol−1, which again agrees well with the calculated energetic span of + 80.8 kJ mol−1 that can be deducted from the Gibbs free energy profile (Figure 1) and the apparent barrier from VTNA analysis (vide supra).
Exo-selective Approach With Catalyst C7
Since the magnetic measurements of the activated catalyst C7 in solid state and solution (vide supra) combined with 1H-NMR spectra suggest the presence of a low-spin Ni(II) complex as largely dominating species which, however, also contains a paramagnetic high-spin Ni(II) fraction, it was unclear which species is catalytically active. Hence, we studied central reaction intermediates on both the singlet and triplet PES (see Supporting Information). Our calculations indicate that the azomethine ylide coordination as a bidentate ligand to the catalyst is strongly endergonic on the singlet PES, while that step is only slightly uphill on the triplet PES. Moreover, the calculated iminoester deprotonation barrier in the singlet case is +111.8 kJ mol−1, which contrasts to the found triplet barrier of + 58.9 kJ mol−1 and is too high for the experimental reaction conditions and outcome. Thus, it is very likely that the minor high-spin fraction of the Ni(II) complex is the catalytically active species.
The predicted Gibbs free energy reaction profiles for the main exo- and minor endo-product 3bA with thermodynamic driving forces of ΔrG° = −62.1 and −54.0 kJ mol−1, respectively, are shown in Figure 3. In case of C7, the iminoester coordination to the catalyst is exergonic. The subsequent proton shift to the aryloxide moiety of the catalyst is endergonic, but has a comparatively low barrier. In contrast to the analogous Co(II)-species derived from C4, the Ni(II) center in IINi (and IIINi as well as IVNi) adopts a square pyramidal geometry by coordination of both the ligand and the ylide.
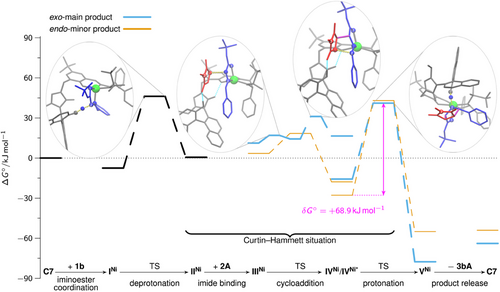
Again, imide binding to IINi is endergonic. From imide adduct IIINi C,C-bond formations occur in either concerted or stepwise manner, depending on the stereoisomer formed. To further investigate this result, 2D PES scans were performed for both possible exo enantiomers, varying both C─C bond lengths systematically (Supporting Information). It was found that only in the case where stabilization of a potential Michael intermediate by hydrogen bonding between it and the naphthol-OH group is possible, a stepwise C,C-bond formation can be achieved. In all other cases, an asynchronous concerted mechanism is observed. The described stabilization is only possible for one of the two exo-product enantiomers, which is the main product using C7. In that case, in the TSs of both C,C-bond formation steps (Figure 4a,b), the C–H acidic triazolium moiety forms a hydrogen bond with the phenoxy moiety of the ligand in addition to the hydrogen bond activation of the maleimide. Similarly to the concerted C,C-bond formation with C4 (Figure 2a), this serves as a structural anchor to stabilize the spatial position of the azolium / naphthol moiety.
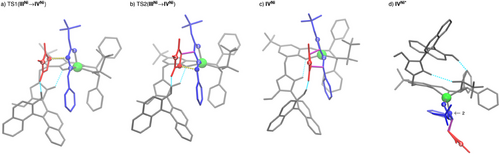
After C,C-bond formation, also IVNi (Figure 4c) needs to undergo a conformational change to form IVNi*, which is stabilized by a hydrogen bond between the naphthol OH group and the sulfonyl motif before protonation of the cycloadduct can take place. This change also results in a strong twisting of the azolium/naphthol moiety. In IVNi*, it is possible to bind the naphthol OH to the Ni(II) center in an octahedral coordination geometry, allowing protonation of the exo-configured cycloadduct (Figures 3 and 4d). Protonation of the minor endo-isomer follows the same mechanism. Regeneration C7 by product release closes the catalytic cycle. That dissociation is calculated to be endergonic for both exo and endo products.
Due to the low barrier of the C,C-bond formations, they are reversible for both the exo and the endo reaction paths. In addition, the barrier of the cycloadduct protonation from IVNi* is higher than the barrier of the back-reaction to IIINi. This results in a Curtin–Hammett situation, which means that IVNi* (exo) and IVNi*(endo) are in equilibrium with each other. As the barrier to cycloadduct protonation is lower for the exo-product than for the endo-product, the exo-product is preferentially formed.
Based on our computational model, the exo:endo-ratio is determined by the Gibbs free energy difference between the protonation TSs, i.e., TS IVNi* → VNi. This value is found to be sensitive to the DFT functional used (see Supporting Information). B3LYP-D3(BJ)/def2-TZVP/COSMO(THF) yields a value of about 68:32. Using COSMO-RS[103-105] instead of COSMO[106-108] as an implicit solvent mode predicts an exo:endo-ratio of 88:12 (experimental: 92:8).
Conclusions
In summary, we have developed a new catalyst concept for 1,3-DCA, which allows selective access to both the endo and exo diastereomers on demand from azomethine ylides and maleimides. Two highly productive polyfunctional catalysts were found, each offering a mode of action that is reminiscent of enzymatic catalysis in various aspects.
For the endo-selective 1,3-DCA with C4, the combination of experimental and computational mechanistic studies shows that the high diastereo- and enantioselectivity, as well as the excellent productivity, can be attributed to a nearly perfect spatial fit between the functional groups of the catalyst and the functional units of both reaction partners throughout the whole catalytic cycle. Once the limiting activation barrier for deprotonation is overcome, a simple rotation around the CH₂ unit, bridging the metal-sphere and the azolium/naphthol motif, enables the functional groups of the catalyst to be arranged in a nearly optimal position for all individual steps of the catalytic cycle, in particular for the low-barrier concerted cycloaddition event. The resulting structures of the catalytic cycle are stabilized by the interplay of hydrogen bonding, allowing for a cooperative mode of substrate activation. This shows a strong analogy to the working principles of enzymes.
To enable the desired switch in diastereoselectivity, it is necessary to hamper this main, inherently preferred pathway. This can be achieved by changing the metal center from Co(II) to Ni(II), thus changing the preferred metal coordination sphere, while also varying the azolium moiety (to facilitate twisting of the azolium / naphthol moiety) and increasing the steric demand of the ester residue of the iminoester (Et vs. tBu).
In case of the exo-selective catalysis with C7, the modified coordination polyhedron of the Ni(II) center enables the formation of a structurally well-defined binding pocket after deprotonation of the iminoester by forming a hydrogen bond between the naphthol group and the sulfonyl unit. As a result, the acidic proton of the naphthol group is in close proximity to the pyrrolidinate nitrogen atom, which enables a facilitated proton transfer. The selectivity is a result of a Curtin–Hammett situation and is strongly dependent on steric interactions.
This interdisciplinary study demonstrates the large potential of the modular polyfunctional Lewis acid/azolium aryloxide catalysts in terms of productivity and well-defined stereocontrol. The mode of action is comparable to an enzymatic machinery, which is capable of performing and controlling a number of sequential events in a very precise manner. The efficiency of these systems culminates in a facilitation of the actual key step in such a way that their barrier is lower than that of the seemingly more trivial steps. However, as the reported catalyst systems are also capable of controlling and accelerating these steps, their efficiency is without precedent.
Acknowledgements
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG, project ID 310990893 – PE 818/7–2; project ID 358283783 – CRC 1333/2). The authors acknowledge support by the state of Baden–Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575–1 FUGG (JUSTUS 2 cluster). P.M.B. acknowledges financial support in the form of a Ph.D. scholarship from the Studienstiftung des deutschen Volkes (German National Academic Foundation).
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.