Orbital Coupling-Engineered Coordination-Unsaturated CuAg Nanochains Drives Spontaneous Electrocatalytic Acetylene Semihydrogenation and Zn-C2H2 Batteries
Graphical Abstract
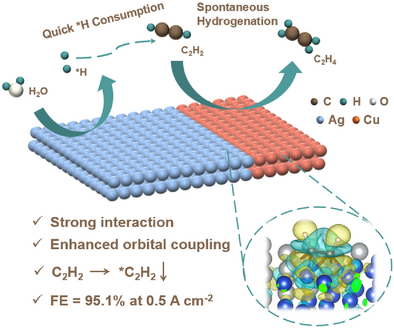
Coordination unsaturated Cu-Ag cross-linked nanochains (Cu0.5Ag CNCs) is synthesized for ethylene electrocatalytic production, achieving an ethylene Faradaic efficiency of 95.1% at 0.5 A cm−2. The orbital coupling between the Cu0.5Ag CNCs catalyst and the C2H2 molecules is enhanced and forms a high *H demand surface, where the acetylene hydrogenation process occurs spontaneously without energy barrier. It provides a new way to realize the orbital coupling engineering of coordination unsaturated bimetallic catalysts.
Abstract
Electrocatalytic semihydrogenation of acetylene (C2H2) offers a mild and sustainable pathway for ethylene production, yet it faces critical challenges including competitive C─C coupling for 1,3-butadiene due to insufficient proton supply and hydrogen evolution under high current densities. To address these limitations, we design a coordination-unsaturated CuAg bimetallic catalyst with cross-linked nanochains (Cu0.5Ag CNCs), which synergistically regulates proton dynamics, maintaining high activity from 0.1 to 0.6 A cm−2 and achieving an ethylene Faradaic efficiency of 95.1% at 0.5 A cm−2. Mechanistic studies reveal that the introduction of Cu makes the d-band center in Cu0.5Ag CNCs upshift toward the Fermi level, strengthening orbital coupling with C2H2 and creating a high *H demand surface. In situ spectroscopic and density functional theory analyses demonstrate that coordination-unsaturated Cu-Ag interfacial sites promote spontaneous C2H2 hydrogenation, especially bypassing formation barriers of *C2H2 and *C2H3. This thermodynamic superiority originates from sufficient proton supply and exothermic *H consumption for C2H2 hydrogenation. Furthermore, the catalyst enables a Zn-C2H2 battery with a power density of 2.12 mW cm−2, showcasing dual functionality in electrosynthesis and energy storage. Our work establishes a paradigm for coordination unsaturated bimetallic catalyst design through orbital coupling engineering, providing atomic-level insights into proton-mediated reaction control for sustainable chemical manufacturing.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.