Sequential Reconstruction of Calicheamicin γ1I Iodo-Thiobenzoate by Selective Carrier Protein Trapping Reveals a Flavin-Dependent Iodinase
Graphical Abstract
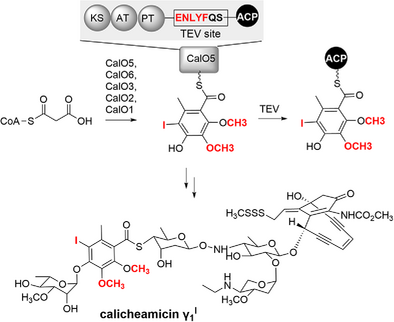
Enzymatic synthesis of the iodo-thiobenzoate of calicheamicin γ1I was reconstituted in vitro from malonyl-CoA, involving the orsellinate biosynthesis by CalO5 and subsequent modifications by CalO6, CalO3, CalO2, and CalO1. A TEV site was introduced into CalO5, enabling the successful detection of the intermediate or product attached to the ACP domain through intact-protein mass spectrometric analysis.
Abstract
Flavin-dependent halogenases (FDHs) play important roles in natural product biosynthesis, particularly chlorinases and brominases. Apart from ubiquitous mammalian iodination in thyroxine biosynthesis, iodinated natural products are extremely rare, and enzymes responsible for iodination are even less well described. A notable exception is calicheamicin γ1I, a potent antitumor compound containing an aromatic iodide, for which a halogenase has been proposed to mediate iodine incorporation. Despite predictions regarding the enzymes involved in iodinated aryl ring biosynthesis, experimental evidence remains limited due to challenges in substrate identification and reaction monitoring. In this study, we successfully reconstituted the enzymatic activities required for iodination and all embellishments of the highly substituted benzene ring in calicheamicin γ1I. Using intact-protein mass spectrometry combined with protease cleavage, we demonstrated formation of the orsellinate thioester followed by sequential C-2 O-methylation, C-5 iodination, C-3 oxidation, and C-3 O-methylation. This research characterizes the first flavin-dependent iodinase that acts on a carrier protein-dependent substrate, identifies the natural substrates of a cytochrome P450 oxygenase and two O-methyltransferases, and provides valuable insights for biocatalysis. Additionally, these findings could facilitate the engineering of other polyketide biosynthetic pathways and contribute to optimizing fermentation conditions to generate new calicheamicin derivatives.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.