Introducing Steric Bulk Into Silylboranes: Enhanced Bench Stability and Novel Chemical Reactivity
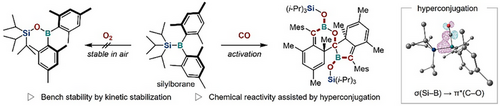
Graphical Abstract
Following initial experimental and theoretical studies that indicated that silylboranes decompose via oxygenation, bulky silyl anions were used to synthesize silylboranes with enhanced bench stability and novel reactivity. These kinetically stabilized silylboranes are stable in air and moderately activate carbon monoxide (CO) to form spirocyclic compounds. DFT calculations suggested that the activation of CO is driven by hyperconjugation.
Abstract
Silylboranes are versatile intermediates in organic synthesis, and a wide range of structural variants of silylboranes have already been synthesized. However, the stability of silylboranes varies significantly, and their decomposition mechanism remains to be fully understood. Moreover, some silylborane motifs have not yet been applied as synthetic reagents due to their instability. To address this issue, we first investigated the decomposition mechanism of silylboranes in air and moisture using experimental and theoretical methods. We discovered that oxygenation by atmospheric oxygen is the major decomposition pathway, resulting in the formation of a borylsilylether, and that the introduction of a bulky silyl group suppresses this decomposition. Based on these results, we synthesized triisopropylsilyldimesitylborane (i-Pr3Si–BMes2), which exhibits high bench stability. Moreover, i-Pr3Si–BMes2 reacts with carbon monoxide (CO), which is the first example of a reaction between a silylborane and CO. Further computational studies revealed that electronic effects, including hyperconjugation between the Si─B σ-bond and C─O π*-bond, are crucial for CO activation. Furthermore, we prepared a bench-stable bissilylaminoborane by introducing triisopropylsilyl groups, providing a bissilylchloroborane upon hydrogen chloride treatment. These findings provide valuable insights into how steric and electronic effects can be used to optimize the balance between stability and reactivity in silylboranes.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.