EDTA-Mg Nano-Chelators Amplify Ferroptosis by Artificially Simulating the Epithelial-Mesenchymal Transition Process and Endogenous Iron Deprivation
Graphical Abstract
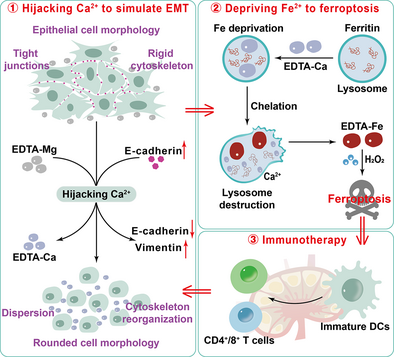
Based on metal chelation therapy, we utilize the chelation performance of ethylene diamine tetraacetic acid (EDTA) for different divalent metal ions to achieve the precise regulation of the epithelial-mesenchymal transition (EMT) process in tumors and simultaneously synergize ferroptosis and immunotherapy to effectively inhibit the growth and metastasis of tumors.
Abstract
Epithelial-mesenchymal transition (EMT) is a key step in initiating tumor metastasis. Commonly, researchers focus on inhibiting EMT to prevent tumor metastasis. However, they ignore that tumor cells undergoing EMT are more vulnerable to disturbance from the external environment. Tumor cells in this period are a potential therapeutic target, yet precisely regulating the EMT of tumor cells remains a challenging problem to be solved. Here, based on metal chelation therapy, we propose a strategy of artificially mimicking EMT, integrating ferroptosis and immunotherapy to inhibit tumor growth and metastasis. The prepared ethylene diamine tetraacetic acid-magnesium (EDTA-Mg), on the one hand, chelates Ca2+ on the surface of tumor cells to form EDTA-Ca, causing the dissociation of tumor cells. Meanwhile, E-cadherin is downregulated, while Vimentin and matrix metalloproteinase 2 (MMP-2) are upregulated, indicating the occurrence of EMT. On the other hand, after EDTA-Ca is endocytosed by tumor cells, it deprives Fe in the lysosomes to form EDTA-Fe, which induces ferroptosis through a Fenton reaction. Ferroptosis, combined with the initially released Mg2+, synergistically amplifies the immune response, thereby inhibiting tumor metastasis. To the best of our knowledge, such a strategy of artificially simulating EMT for tumor treatment has hitherto not been reported.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.