Electron-Delocalized Cu2+ Activates Spin Channels in Spinel Oxides to Selectively Produce 1O2 for Wastewater Treatment
Le-Yang Hao
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Both authors contributed equally to this work.
Search for more papers by this authorZi-Jun Tang
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Both authors contributed equally to this work.
Search for more papers by this authorChu-Yi Cai
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorYu-Chen Zhao
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Lei Tian
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorProf. Nan Li
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Zhao-Qing Liu
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
School of Chemistry, South China Normal University, Guangzhou, 510006 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorLe-Yang Hao
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Both authors contributed equally to this work.
Search for more papers by this authorZi-Jun Tang
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Both authors contributed equally to this work.
Search for more papers by this authorChu-Yi Cai
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorYu-Chen Zhao
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Lei Tian
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorProf. Nan Li
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Zhao-Qing Liu
School of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials/Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou, 510006 P.R. China
School of Chemistry, South China Normal University, Guangzhou, 510006 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
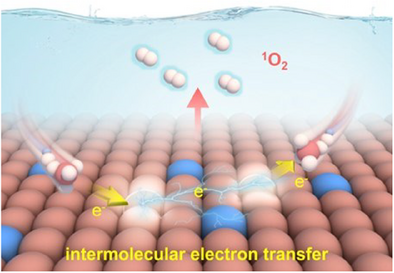
This work proposes an electron-delocalized strategy to unlock FeTd2+─O─FeOh3+ spin channel, inducing intermolecular electron transfer of two PMS for selective 1O2 generation. The directed electron transfer behavior not only maintains the catalyst stability, but also immensely reduces the 1O2 generation energy barrier and improves the PMS utilization.
Abstract
Blocked electron transfer in the catalyst during advanced oxidation processes causes sluggish singlet oxygen (1O2) generation efficiency and sacrifices catalyst stability. In this work, we propose an electron-delocalization strategy that unlocks ATd2+─O─BOh3+ electron-transfer pathways within spinel oxide (Cu0.8Fe2.2O4), inducing the intermolecular electron transfer of peroxymonosulfate (PMS) for selective 1O2 generation. In situ characterizations and theoretical calculations confirm that the electron-delocalized Cu2+ triggers a high spin-state of O in FeTd2+─O─FeOh3+, thus creating a spin channel for the spontaneous intermolecular electron transfer of PMS from the FeOh3+ adsorption site to the FeTd2+ adsorption site through FeTd2+─O─FeOh3+. This process allows for the simultaneous oxidation and reduction of PMS, thereby reducing the energy barriers for the formation of SO4•− and SO5•− radicals. Subsequently, the interfacial SO4•− rapidly oxidizes SO5•− into 1O2, enhancing 1O2 generation efficiency without sacrificing catalyst stability. The selectivity of 1O2 in the Cu0.8Fe2.2O4/PMS system reaches 98.4%. Multiple pollutants are removed in the Cu0.8Fe2.2O4/PMS system without interference from coexisting substances. The scale-up experiment realizes 100% contaminant removal during the continuous operation process (48 h). This work exhibits a novel strategy for selective 1O2 generation to achieve the goal of practical applications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202504426-sup-0001-SuppMat.docx22.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Q. Tang, B. Wu, X. Huang, W. Ren, L. Liu, L. Tian, Y. Chen, L. S. Zhang, Q. Sun, Z. Kang, T. Ma, J. P. Zou, Nat. Commun. 2024, 15, 9549.
- 2J. Tian, B. Li, C. Wu, Z. Li, H. Tang, W. Song, G. B. Qi, Y. Tang, Y. Ping, B. Liu, J. Am. Chem. Soc. 2024, 146, 16458–16468.
- 3T. Lei, Y. Y. Cheng, X. Han, C. Zhou, B. Yang, X. W. Fan, B. Chen, C. H. Tung, L. Z. Wu, J. Am. Chem. Soc. 2022, 144, 16667–16675.
- 4T. Liu, S. Xiao, N. Li, J. Chen, X. Zhou, Y. Qian, C. H. Huang, Y. Zhang, Nat. Commun. 2023, 14, 2881.
- 5Z. Wu, B. Huang, X. Wang, C. S. He, Y. Liu, Y. Du, W. Liu, Z. Xiong, B. Lai, Environ. Sci. Technol. 2023, 57, 14046–14057.
- 6M. Huang, Y. S. Li, C. Q. Zhang, C. Cui, Q. Q. Huang, M. Li, Z. Qiang, T. Zhou, X. Wu, H. Q. Yu, Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2202682119.
- 7Z. Liu, R. Su, F. Xu, X. Xu, B. Gao, Q. Li, Adv. Mater. 2024, 36, 2311869.
- 8D. Zhang, Y. Li, P. Wang, J. Qu, Y. Li, S. Zhan, Nat. Commun. 2023, 14, 3538.
- 9A. Wang, B. Z. Zhu, C. H. Huang, W. X. Zhang, M. Wang, X. Li, L. Ling, J. Ma, J. Fang, Water Res. 2023, 235, 119904.
- 10S. Sun, C. Shan, Z. Yang, S. Wang, B. Pan, Environ. Sci. Technol. 2022, 56, 634–641.
- 11X. Jiang, B. Zhou, W. Yang, J. Chen, C. Miao, Z. Guo, H. Li, Y. Hou, X. Xu, L. Zhu, D. Lin, J. Xu, Proc. Natl. Acad. Sci. U.S.A. 2024, 121, e2309102121.
- 12Z. Y. Guo, Y. Si, W. Q. Xia, F. Wang, H. Q. Liu, C. Yang, W. J. Zhang, W. W. Li, Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2201607119.
- 13Z. Weng, Y. Lin, S. Guo, X. Zhang, Q. Guo, Y. Luo, X. Ou, J. Ma, Y. Zhou, J. Jiang, B. Han, Angew. Chem., Int. Ed. 2023, 62, e202310934.
- 14W. Ren, C. Cheng, P. Shao, X. Luo, H. Zhang, S. Wang, X. Duan, Environ. Sci. Technol. 2022, 56, 78–97.
- 15J. Zhu, F. Yu, J. Meng, B. Shao, H. Dong, W. Chu, T. Cao, G. Wei, H. Wang, X. Guan, Environ. Sci. Technol. 2020, 54, 9702–9710.
- 16Y. Gao, Z. Chen, Y. Zhu, T. Li, C. Hu, Environ. Sci. Technol. 2020, 54, 1232–1241.
- 17P. Shao, Y. Jing, X. Duan, H. Lin, L. Yang, W. Ren, F. Deng, B. Li, X. Luo, S. Wang, Environ. Sci. Technol. 2021, 55, 16078–16087.
- 18L. S. Zhang, X. H. Jiang, Z. A. Zhong, L. Tian, Q. Sun, Y. T. Cui, X. Lu, J. P. Zou, S. L. Luo, Angew. Chem., Int. Ed. 2021, 60, 21751–21755.
- 19G. Bi, R. Ding, J. Song, M. Luo, H. Zhang, M. Liu, D. Huang, Y. Mu, Angew. Chem. Int. Ed. 2024, 63, e202401551.
- 20Y. Yao, C. Wang, X. Yan, H. Zhang, C. Xiao, J. Qi, Z. Zhu, Y. Zhou, X. Sun, X. Duan, J. Li, Environ. Sci. Technol. 2022, 56, 8833–8843.
- 21L. Tian, Z. J. Tang, L. Y. Hao, T. Dai, J. P. Zou, Z. Q. Liu, Angew. Chem. Int. Ed. 2024, 63, e202401434.
- 22C. Wang, B. H. Xiao, J. Huang, K. Xiao, Z. Q. Liu, Adv. Funct. Mater. 2024, 34, 2405680.
- 23K. Xiao, Y. Wang, P. Wu, L. Hou, Z. Q. Liu, Angew. Chem. Int. Ed. 2023, 62, e202301408.
- 24J. L. Peng, Y. L. Luo, J. X. Li, J. L. Huang, B. Xiao, C. F. Xiao, K. Xiao, Z. Q. Liu, Nano Lett. 2024, 24, 1687–1694.
- 25H. T. Tang, Z. J. Tang, R. L. Li, L. Tian, R. Wang, S. Pu, Z. Q. Liu, Appl. Catal. B Environ. Energy. 2024, 358, 124436.
- 26L. Tian, X. Y. Xia, L. J. Zhou, L. L. Zheng, Q. J. Xing, L. S. Zhang, J. P. Zou, Z. Q. Liu, Appl. Catal. B Environ. 2024, 340, 123260.
- 27L. Ye, L. Zhou, Y. Lu, Ind. Eng. Chem. Res. 2022, 61, 4320–4328.
- 28H. Dong, W. Du, J. Dong, R. Che, F. Kong, W. Cheng, M. Ma, N. Gu, Y. Zhang, Nat. Commun. 2022, 13, 5365.
- 29Q. Ji, Y. Kong, H. Tan, H. Duan, N. Li, B. Tang, Y. Wang, S. Feng, L. Lv, C. Wang, F. Hu, W. Zhang, L. Cai, W. Yan, ACS Catal. 2022, 12, 4318–4326.
- 30L. Gao, C. Tang, J. Liu, L. He, H. Wang, Z. Ke, W. Li, C. Jiang, D. He, L. Cheng, X. Xiao, Energy Environ. Mater. 2021, 4, 392–398.
- 31M. M. Huie, D. C. Bock, L. Wang, A. C. Marschilok, K. J. Takeuchi, E. S. Takeuchi, J. Phys. Chem. C. 2018, 122, 10316–10326.
- 32J. Zhou, F. Qiao, Z. Ren, X. Hou, Z. Chen, S. Dai, G. Su, Z. Cao, H. Jiang, M. Huang, Adv. Funct. Mater. 2024, 34, 2304380.
- 33L. Tian, L. S. Zhang, L. L. Zheng, Y. Chen, L. Ding, J. P. Fan, D. S. Wu, J. P. Zou, S. L. Luo, Angew. Chem. Int. Ed. 2022, 61, e202214145.
- 34L. Tian, P. Chen, X. H. Jiang, L. S. Chen, L. L. Tong, H. Y. Yang, J. P. Fan, D. S. Wu, J. P. Zou, S. L. Luo, Water Res. 2022, 209, 17890.
- 35Y. Lei, Y. Yu, X. Liang, S. S. Cheng, G. Ouyang, X. Yang, Environ. Sci. Technol. 2023, 57, 5433–5444.
- 36L. Tian, M. Y. Yin, L. L. Zheng, Y. Chen, W. Liu, J. P. Fan, D. S. Wu, J. P. Zou, S. L. Luo, Sep. Purif. Technol. 2023, 309, 123021.
- 37W. Zheng, L. Zhu, S. Liang, J. Ye, X. Yang, Z. Lei, Z. Yan, Y. Li, C. Wei, C. Feng, Environ. Sci. Technol. 2020, 54, 9015–9024.
- 38K. Wang, J. Dai, G. Zhan, L. Zhao, R. Wang, X. Zou, J. Wang, Q. Zheng, B. Zhou, R. Zhao, Y. Zhang, W. Lian, Y. Yao, L. Zhang, Angew. Chem. Int. Ed. 2024, 63, e202412209.
- 39R. Zhao, Z. Zhu, T. Ouyang, Z. Q. Liu, Angew. Chem. Int. Ed. 2024, 136, e202313597.
- 40J. Cai, H. Zhang, L. Zhang, Y. Xiong, T. Ouyang, Z. Q. Liu, Adv. Mater. 2023, 35, 2303488.
- 41Y. Sun, X. Ren, S. Sun, Z. Liu, S. Xi, Z. Xu, Angew. Chem. Int. Ed. 2021, 133, 14657–14665.
- 42H. Li, P. Shi, L. Wang, T. Yan, T. Guo, X. Xia, C. Chen, J. Mao, D. Sun, L. Zhang, Angew. Chem. Int. Ed. 2023, 62, e202216268.
- 43S. Suleman, Y. Zhang, Y. Qian, J. Zhang, Z. Lin, Ö. Metin, Z. Meng, H. L. Jian, Angew. Chem. Int. Ed. 2024, 63, e202314988.
- 44J. Cheng, S. Wan, S. Cao, Angew. Chem. Int. Ed. 2023, 62, e202310476.
- 45Y. Gao, Y. Zhu, L. Lyu, Q. Zeng, X. Xing, C. Hu, Environ. Sci. Technol. 2018, 52, 14371–14380.
- 46M. Tian, X. Ren, S. Ding, N. Fu, Y. Wei, Z. Yang, X. Yao, Environ. Res. 2024, 243, 117848.
- 47W. Zheng, Y. Chen, H. Fu, Z. Yan, Z. Lei, W. Duan, C. Feng, Water Res. 2022, 225, 119143.
- 48Z. Feng, M. Chen, Q. Yang, Z. Wang, L. Li, H. Zhao, G. Zhao, Environ. Sci. Technol. 2023, 57, 17123–17131.
- 49W. Xiao, A. Chen, M. Cheng, W. Xiong, Y. Liu, J. Wang, G. Wang, G. Zhang, L. Li, H. Liu, Q. Shi, Water Res. 2025, 268, 122735.