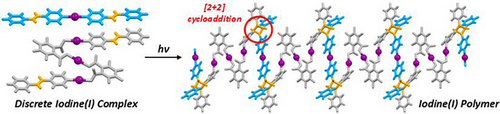
Solid-State Photoconversion of a Discrete Mixed Iodine(I) System to a 1D Polymer
Graphical Abstract
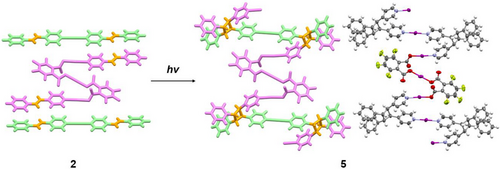
The first example of a mixed halogen(I) complex, which contained three distinct iodine(I) moieties, was synthesized using 4-styrylpyridine and 3,4,5,6-tetrafluorophthalate. This complex underwent a single-crystal-to-single-crystal transformation when exposed to UV light, converting the discrete salt to a 1D polymer while retaining the iodine(I) moieties and represents the only example of a nondestructive photoconversion of a halogen(I) complex.
Abstract
The first example of a mixed halogen(I) complex (2), containing three distinct iodine(I) moieties ([N―I―N]+, O―I―N, and [O―I―O]−) within the same structure, was synthesized with 4-styrylpyridine (4-stypy) and 3,4,5,6-tetrafluorophthalate as the stabilizing Lewis bases. This complex was observed to be in equilibrium with its respective bis(OIN) complex (1a), with isolated samples of 2 also being found to convert to 1a in solution. Upon UV irradiation of 2, a single-crystal-to-single-crystal [2 + 2] cycloaddition reaction was observed, converting the discrete salt 2 to the 1D polymer 5. Complex 5 retained all the iodine(I) moieties from prior to photoconversion and represents the first example of nondestructive photoconversion of a halogen(I) complex. To facilitate comparisons to 2 and 5, several additional closely related iodine(I) complexes were synthesized, with the iodine(I) complexes characterized by NMR (1H, 1H-15N HMBC) and SCXRD, as well as by Raman and IR spectroscopy for 2, 5, and their close structural analogue 1a.
Introduction
The study of halogen bonding, defined as the attractive interaction observed between the electrophilic region (σ-hole)[1] of a halogen atom and a neutral or anionic nucleophile,[2] has only intensified in the last two decades. This continued interest has been supported by the myriad of host–guest systems successfully employing halogen bonding for a range of applications, such as in porous,[3-6] phosphorescent,[7-9] responsive,[10-16] magnetic,[17-19] shape-memory,[20, 21] and liquid crystalline materials,[18, 22-24] for purposes like chemicals separations and ion-pair recognition,[15, 25-30] with catalysis also emerging more recently.[31, 32] As a result, halogen bonding is of interest to a wide variety of researchers from a multidisciplinary array of scientific backgrounds.
One subfield of halogen bonding that has significantly established itself in recent decades is the subset of species incorporating a fully ionized halogen(I) ion (also termed a halenium ion),[33] X+ (X = I, Br, Cl).[34] When coordinated to a pair of stabilizing Lewis bases (L), these halogen(I) ions can be isolated as comparatively stable halogen(I) complexes (also known as halonium, or more specifically for iodine(I) derivatives, iodonium complexes).[35] These halogen(I) complexes are some of the strongest halogen bond donors known and benefit from a high degree of linear directionality in their bonding,[35, 36] a result of their electronic origin as a σ-hole interaction, which means that the strength of their bonding follows the general trend: I > Br >> Cl.[1] The stabilizing Lewis bases are most commonly aromatic N-heterocycles, with the most famous of such species being the eponymous Barluenga's reagent, [I(py)2]BF4 (py = pyridine).[37] These [N―I―N]+ complexes were first identified in the 1960s (Figure 1),[38] but were popularized in the late 1980s by Barluenga and co-workers due to their pioneering work demonstrating a wide array of uses in organic transformations as a mild iodination and oxidizing reagent.[39-41] In addition to their reactivity, the highly directional halogen(I) centers have also found extensive success in assembling supramolecular architectures and coordination networks,[3, 4, 42-45] most recently demonstrated to extend to bromine(I) examples as well,[5, 46] and for which applications have already begun to emerge.[6, 47]
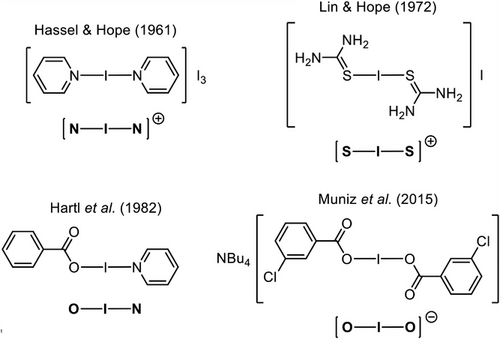
In the same vein, heteroleptic iodine(I) complexes of the form O―I―N were first reported in 1981 by Hartl et al. (Figure 1),[49] with research in this area having only been recently rekindled due to the structurally confirmed similarity of these O―I―N complexes to the iodine(I) [N―I―N]+ complexes.[51-58] This revitalization is apparent in the number of solid-state examples of O―I―N iodine(I) complexes, which has skyrocketed in 2024 (56 total), more than doubling the number reported up to 2023 (23).[59] Computational studies to calculate the molecular electrostatic potential surfaces (MEPS) of some iodine(I) complexes have indicated that the F3CC(O)O–I fragment, which could not be experimentally isolated, is the strongest halogen bond donor known to date with a calculated σ-hole of +234.6 kJ mol−1, even greater than that calculated for N-iodosaccharin (+226.4 kJ mol−1).[51] The predominant motif for reported O―I―N complexes is as iodine(I) carboxylates (also termed carbonyl hypoiodites, or simply, hypoiodites), RC(O)O―I―L, largely due to the abundance of carboxylic acids that constitute the starting materials for their subsequent synthesis. In 2024, however, two exotic subclasses were also reported: R2P(O)O―I―L and RS(O)2O―I―L,[60, 61] both with wildly different properties. The iodine(I) phosphinates (R2P(O)O―I―L) were found to be isolable with the same relative water tolerance as their iodine(I) carboxylate analogues but with significantly improved reactivity as iodination reagents.[60] In contrast, the iodine(I) sulfonates (RS(O)2O―I―L) could only be kinetically trapped at subzero temperatures, with rapid decomposition within seconds observed under ambient conditions that, unfortunately, has hindered any subsequent reactivity studies.[61]
The smallest subset of iodine(I) complexes is those comprised of homoleptic chalcogen donor atoms, either S or O. The [S―I―S]+ motif dates back to 1972, with neutral thiourea acting as the Lewis base (Figure 1),[48, 59] with only sporadic reports of fresh examples since then to a current total of 14, and it should be noted that there are no analogous examples with phosphorus for the [N―I―N]+ motif. Lastly, the [O―I―O]− motif (termed dioxoiodanes by Muñiz)[62] incorporates anionic carboxylates as the stabilizing Lewis bases (Figure 1), for which there are only a modest number of solid-state examples in the literature (7 in total, not including examples that only differ in the cation).[50, 62-64] Examples of [O―I―O]+ have also been reported with neutral oxygen-donor Lewis bases,[65] but these were not observed in the solid state.
The use of light as a stimulus to effect chemical reactions has been well-established in organic photochemistry;[66, 67] however, emerging solid-state cycloaddition chemistry has seen significant progress in the development of functional materials with a wide range of applications.[68-70] One notable photochemical reaction in such solids is the [2 + 2] cycloaddition, where two alkenyl groups react under light irradiation in a highly specific and potentially reversible reaction; the formation of a cyclobutane ring that results makes it a powerful tool for designing light-responsive materials. Similar strategies have been widely explored to develop photo-responsive organic compounds, polymers, and coordination complexes, as well as the closely related metal–organic frameworks.[71-75] Nonetheless, reports on halogen-bonded complexes displaying optical and/or photo-responsive properties remain scant.[11-14, 16, 23, 76, 77] The paucity of examples based on halogen(I) complexes is even more notable[78] and is likely due to the challenges associated with the reactivity of these complexes and, more importantly, establishing the prerequisite geometric conditions in crystalline solids to facilitate the photochemical reactivity or multifunctionality in these systems.
Results and Discussion
Building upon previous reports of bis(OIN) complexes utilizing dicarboxylate-based backbones with various substituted pyridines,[52, 57] the analogous incorporation of 4-styrylpyridine (4-stypy) was envisioned as a possible route to photoactive iodine(I) complexes. Photo-responsive halogen-bonded materials are known,[11-14, 16] but there is only a single prior study exploring a photo-responsive halogen(I) complex,[78] which involved a light-induced cis/trans isomerization of an alkenyl group within an iodine(I) complex. This ultimately proved destructive to the iodine(I) center within the complex being tested due to the rearrangement of the structure, though it was confirmed that the analogous iodine(I) complex without the alkenyl group did not degrade under the same photo-irradiation conditions.[78] The resulting bis(OIN) complex incorporating 4-stypy (1a; Scheme 1) was synthesized by a one-pot reaction of silver(I) tetrafluorophthalate (TFP), 4-stypy (2 equiv.), and elemental iodine (2 equiv.). Given the prior reports of interactions between iodine(I) and silver(I) centers (I+⋯Ag+) in the solid state,[33, 79, 80] the identical reaction was also performed with one equivalent of iodine(I), in an attempt to produce a mixed mono(OIN)-mono(OAgN) complex. However, upon slow evaporation of the filtered reaction mixture in CH2Cl2, crystals of the mixed multi-iodine(I) NIN/OIN/OIO complex (2) were observed (Scheme 1), in combination with crystals of 1a.
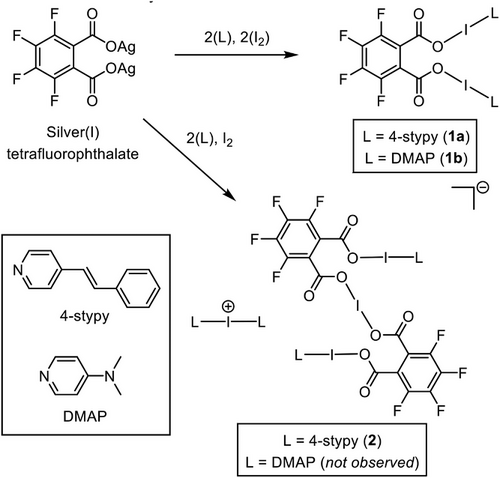
The isolation of 2, the only known example with more than one iodine(I) motif present in a single complex, was unexpected for several reasons: 1) While [N―I―N]+ and O―I―N complexes have been previously shown to have superior water tolerance, [O―I―O]− complexes have only been reported under inert Schlenk conditions.[50, 62] 2) The stoichiometry of the components in 2 is simply double that of 1a, suggesting the synthesis of 1a using two equivalents of I2 should also be able to generate 2, and in theoretically higher yields, given that I2 would be the limiting reagent when only one equivalent is used.
To test if the synthesis of mixed iodine(I) complexes like 2 was a general route, 4-dimethylaminopyridine (DMAP) was instead used as the Lewis base. When DMAP was used, the analogous bis(OIN) complex (1b) could be prepared; however, there was no observation of an analogous mixed iodine(I) complex like 2 for either of the stoichiometries of I2 tested (1 and 2 equiv.). To facilitate better solid-state comparisons with 2, the cation of 2 was also deliberately synthesized as the [PF6]− salt, [I(4-stypy)2]PF6 (3).
Attempts were also made to isolate the silver(I) intermediate from the addition of two equivalents of 4-stypy to silver(I) TFP, which was greatly hindered by the intermediates poor solubility, even in polar solvents like MeCN or (CH3)2SO, which also prevented solution NMR studies. Despite the synthesis of 2 being a result of attempting to generate an intramolecular I+⋯Ag+ interaction, there is no evidence to suggest this interaction was present or played a role in the formation of 2. Nevertheless, crystals containing a 1:3 ratio of silver(I) TFP/4-stypy (4) were isolated, which featured a RC(O)O―Ag―OC(O)R group that may be an intermediate that facilitates the formation of the [O―I―O]− group in 2. Initially 2 was isolated via vapor diffusion using CH2Cl2/pentane, with other solvent combinations also being subsequently screened, though it was found that simply cooling the reaction filtrate (in CH2Cl2) to 250 K provided larger and higher-quality single crystals of 2, which were desirable properties for the subsequent studies. This preference was attributed to the polarity of the CH2Cl2 causing precipitation of 2, shifting the equilibrium to favor its formation under these conditions. Ultimately, it is suspected that the 4-stypy is serendipitously optimal to crystallize well as nonsolvated crystals of 2 (Figure 2).
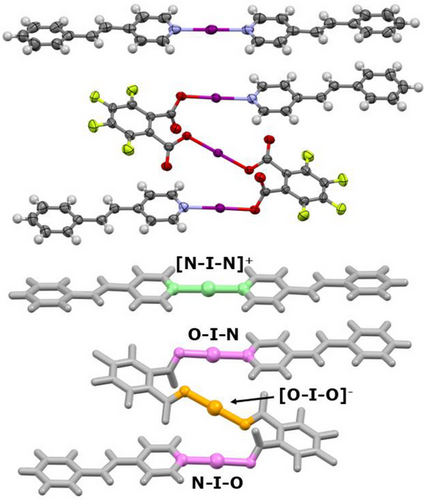
The solid-state structure of 2 determined by SCXRD revealed quite normal iodine(I) bond angles and lengths. The presence of a center of inversion through the [N―I―N]+ and [O―I―O]− iodine(I) centers of 2 meant that only half the complex was crystallographically independent, and that their bond angles were a perfect, symmetry-defined 180°. The O―I―N group was slightly deviated from linear with an angle of 171.9(1)°, but such a deviation had been similarly observed for other O―I―N iodine(I) complexes incorporating fluorinated carboxylates.[52, 57] The bond lengths were also unremarkable in that they mirrored those of their respective, analogous iodine(I) environments (Table 1). As can be seen from Table 1, the bond lengths of the various iodine(I) centers found in 2 are within or just outside a 3σ tolerance of their closest structural analogues, indicating good agreement of the bond lengths for the different iodine(I) complexes when experimental error is taken into account.
| Complex |
I―N ([N―I―N]+) |
I―O/I―N (O―I―N) |
I―O ([O―I―O]−) |
|---|---|---|---|
| 2a) | 2.243(4) | 2.222(3)/2.243(3) | 2.209(2) |
| 3a) | 2.262(3) | – | – |
| 1a·2(CH2Cl2) | – |
2.188(5)/2.265(7) 2.191(6)/2.248(7) |
– |
| [NBu4][I(OC(O)C6H4F-3)2][62]a) | – | – | 2.193(5) |
| 5a) | 2.264(4) | 2.192(9)/2.264(5)b) | 2.201(4) |
- a) Only one set of bond lengths as only half the complex is crystallographically independent due to the presence of a symmetry operation at the iodine(I) center.
- b) The O―I―N group was disordered over two positions, with only the major (64% occupancy) position shown.
Isolated samples of 2 were confirmed to survive under ambient conditions for weeks (as confirmed by SCXRD monitoring of isolated crystals of 2). However, crystals of 2 left in their supernatant CH2Cl2 were observed to convert to 1a after several days. Solution NMR studies confirmed these observations, with isolated crystals of 2 being sluggish to dissolve in (both dry and nontreated) CD2Cl2. The poor solubility of 2 in CD2Cl2 hindered attempts to probe potential temperature and concentration dependence of the solvent with respect to its observed conversion to 1a, with other screened solvents (e.g., CD3CN) promoting the degradation of the iodine(I) centers in 2.
The resulting 1H and 1H-15N HMBC NMR spectra of 2 were always found to be identical to those observed for independently prepared pure samples of 1a, with only a single 15N NMR chemical shift at −167.2 ppm, consistent for an O―I―N iodine(I) complex with a substituted pyridine halogen-bonded to an iodine(I) center. Similarly, the [I(4-stypy)2]+ cation featured in 2, which was individually prepared as the [PF6]− salt (3) and found to have a 15N NMR chemical shift of −182.0 ppm, was absent in all collected spectra of 2, further supporting its conversion to 1a in solution. Unfortunately, because of the observed conversion of 2 to 1a, testing the iodination potential of 2 was abandoned, given the inability to accurately know the structure–reactivity relationship being tested, further complicated by 1a also being expected to demonstrate some reactivity as a source of iodine(I).
The relative receptor capabilities have been probed for the different iodine(I) motifs found in complexes 1a, 1b, 2, and 3 by computational calculations. The interaction energies in the anionic [O―I―O]− are ∼63 kJ mol−1 more favorable than the [N―I―N]+ interactions. By comparison, the neutral O―I―N are substantially weaker than the [N―I―N]+ interaction (cf. 175 kJ mol−1 for [N―I―N]+ in 2 versus 51 kJ mol−1 for O―I―N in 1a) but are relatively unaffected by the nature of the pyridyl ligand. Clearly, a general trend is observed in the interaction energies, but further research is warranted to confirm these preliminary results. The contribution of the lattice potential energies (Upot) should also not be ignored for these ionic solids, which can potentially be exploited to develop new materials.
The comparison of FT-Raman microscopy spectra of crystalline samples of the 4-stypy and H(TFP) ligands, as well as complexes 1a, 2, and 3, provides important information about the influence of the coordination of the iodine(I) center on the vibrational modes of both the 4-stypy and TFP ligands in these complexes (Figures S22–S24). The most prominent Raman modes at 1632 and 1596 cm−1 in these complexes are attributed to the asymmetric (asym) and symmetric (sym) νC═C modes (or combinations thereof) arising from a “quinodal-like” conjugation of the pyridyl and phenyl ring through the alkenyl moiety of the 4-stypy ligand. Other prominent vibrational modes at ∼1198 and ∼1021 cm−1 are attributed to in-plane C‒H scissoring (δ) and rocking (ρ) of the pyridyl, phenyl, and alkenyl hydrogens, and to ring deformation modes of both the pyridyl and phenyl rings, respectively. Surprisingly, the corresponding vibrational and ring deformation modes in neat 4-stypy (cf. 1638, 1580, 1198, and 989 cm−1) are relatively unaffected by the electronic environment produced by the O‒I‒N (1a and 2) or N‒I‒N (2 and 3) coordination and do not significantly affect the intensities of the Raman active modes in these complexes (Figure S22). In contrast, the νC═O modes associated with the TFP ligand in complexes 1b and 2 are significantly shifted (ca. 1694 cm−1) and much less intense in comparison to the neat ligand (cf. νasymC═O 1722 cm−1). This suggests the electrophilic nature of the iodine(I) ion greatly affects the polarization of the carboxylate group in the Raman spectra. Several of the weaker vibrational modes between 550 and 490 cm−1 in 1b and 2 are predominantly attributed to the νO–I constituents, together with ring breathing of the TFP, which are not observed in 3 or the pure 4-stypy. Additionally, a vibrational mode at 463 cm−1 in 2 is assigned to a sym νO–I that arises from the inversion symmetry at the iodine(I) atom in this complex. These tentative assignments are fully supported by DFT analyses (PBE0-D3/def2-TZVP) of the IR and Raman active modes and the visualization of the displacement vectors of all ligands and complexes (see Supporting Information for full details).[81-87] By comparison, the FT-IR spectra of 1a, 2, and 3 are far more complex (Figures S25 and S26) because of the many IR active vibrational modes arising from the TFP ligand. However, a significant shift of the asym νC═O from 1693 cm−1 in the free H(TFP) ligand to ∼1645 cm−1 in both 1a and 2 is similarly found in the IR spectra.
As a general note, no halogenation of the alkene was observed for any of the 4-stypy iodine(I) complexes, similar to the prior observations of a 4-stypy derivative upon reaction with I2 to synthesize liquid crystals.[88] Such halogenation would have been readily apparent in both solution via the loss of the characteristic alkenyl resonances in the 1H NMR spectra, and the solid state due to the huge disparity between the electron counts of hydrogen (Z = 1) and iodine (Z = 53), even for only minimal, partial iodination.
Returning to the initial goal of utilizing the alkene groups of the 4-stypy iodine(I) complexes for [2 + 2] cycloadditions, as had been accomplished in related silver(I) systems,[89-92] only two of the solid-state structures, 1a·2(CH2Cl2) and 2, demonstrated the prerequisite close orientations of a pair of alkene groups. Separate studies had already demonstrated that UV irradiation of [N―I―N]+ groups gave no apparent degradation of the iodine(I) center,[78] though no corresponding information is available for the O―I―N and [O―I―O]− groups. In 1a·2(CH2Cl2), one of the two crystallographically independent O―I―N groups was found to pack head-to-tail with itself by symmetry, with an intermolecular centroid-to-centroid (alkene–alkene) distance of 3.60(1) Å (Figure 3, top). In 2, an off-center overlap of the 4-stypy ligands of the [N―I―N]+ and O―I―N groups aligns their alkene groups in close proximity with a slightly longer centroid-to-centroid distance of 3.96 Å (CH⋯CH = 3.872(7) and 4.042(6) Å; Figure 3, bottom). For both 1a·2(CH2Cl2) and 2, the separation distances of their respective alkene groups are within the viable range for [2 + 2] cycloadditions.[93, 94]
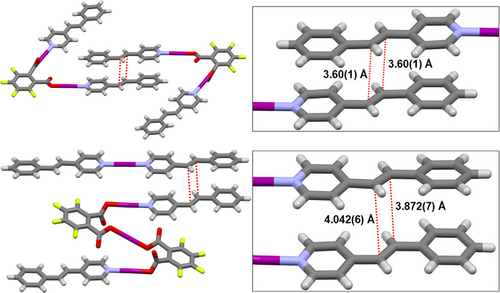
Unfortunately, the solvated nature of 1a·2(CH2Cl2), which was observed to desolvate on a timescale of minutes, was not an ideal candidate for subsequent UV irradiation studies. Nevertheless, UV irradiation experiments were performed on 2 using a Xe lamp (150 W) fitted with a filter to irradiate in the range of 270–380 nm, consistent with prior successful experiments on silver(I) derivatives of 4-stypy.[90]
Upon UV irradiation of 2, tested at intervals of 6 and 21 h, the photoinduced [2 + 2] cycloaddition product 5 was observed via a single-crystal-to-single-crystal transformation (Figure S1). In 5, the newly formed cyclobutane groups obtained by linking both of the alkenyl groups of the [I(4-stypy)2]+ cation to the 4-stypy substituents of two neighboring anions, converted the discrete mixed iodine(I) complex (2) to a 1D polymer (5) with full retention of all the iodine(I) moieties (Figure 4). While this is the only example of a halogen(I) complex undergoing a [2 + 2] cycloaddition, the relative stereochemistry of newly formed cyclobutane groups indicated that the mechanism for this photoconversion proceeded in the same manner as prior literature examples of silver(I) complexes incorporating 4-stypy.[89-92]
Surprisingly, the iodine(I) bond lengths (both I―O and I―N) were observed to be effectively unchanged by the conversion of 2 to 5 (Table 1), with the [N―I―N]+ and [O―I―O]− groups again having symmetry-defined angles of 180°, and the O―I―N angle of 171.8(3)° (for the major disordered position) also being within the expected range. Interestingly, the through-space distances between the iodine(I) atoms (I⋯I) were found to almost perfectly invert, with the I(NIN)⋯I(OIN) and I(OIN)⋯I(OIO) distances in 2 of 4.24 and 4.48 Å, respectively, becoming 4.49 and 4.23 Å in 5, indicating a potential method to modulate these interactions while retaining the iodine(I) moieties. This could potentially be used to generate purpose-specific halogen(I) smart materials in the future, e.g., in light-driven molecular motors or utilizing chirality-induced spin selectivity (CISS) due to the four newly formed chiral carbon atoms of the cyclobutane groups.[95-98]
While Raman spectroscopy is not commonly used to analyze halogen(I) complexes, it has been utilized in the broader field of halogen bonding,[34] as well as for specific purposes like the monitoring of halogen-bond-directed co-crystallization,[99] and was seen as apt for probing the photoconversion of 2 to 5. Comparison of the Raman spectra of 2 and 5 exhibited distinct changes in Raman intensities, which result from the lower symmetry in the latter (Figure 5). The loss of the alkenyl moiety in 2 upon formation of the cyclobutane ring in 5 leads to loss of the “quinodal-like” conjugation of the pyridyl and phenyl ring and results in significant intensity changes to the νC═C modes. Moreover, the removal of the conjugation between the pyridyl and phenyl aromatic rings effectively isolates each ring system, leading to the loss of the distinctive in-plane C‒H scissoring (δ) and rocking (ρ) associated with the fully conjugated aromatic and alkenyl hydrogens found in 2 (vide supra), as well as the appearance of new modes attributed to the isolated pyridyl and phenyl ring systems. A small shift of the νC–H to higher wavenumbers in 5 compared to 2 was observed, consistent with the loss of conjugation and aromatic sp2 hydrogen stretching modes, and the appearance of a relatively weak band at 2952 cm−1 is attributed to the sp3 hydrogen stretching mode of cyclobutane ring. The relatively intense vibrational mode at 1002 cm−1 in the Raman spectra of 5 is tentatively assigned to the in-plane δ mode of the phenyl C─H, while the appearance of two vibrational modes at 772 and 746 cm−1 is attributed to the out-of-plane wagging (ω) or twisting (τ) of the phenyl and pyridyl aromatic C─H.

The increased disorder and distortion in the polymeric structure of 5, arising from the formation of the cyclobutane ring, and the lowering of the symmetry lead to a more complex Raman spectra because of the coupling of vibrational modes. The appearance of many new and weak vibrational modes between 600 and 300 cm−1 is directly related to the loss of the inversion symmetry found in 2 and are likely associated with several different sym and asym combination modes arising from coupling of the O–I–O with the joined O–I–N and N–I–N coordination moieties. This makes the assignment of these specific vibrational modes challenging to distinguish in this polymeric structure compared to 2. Similarly, a comparison of the bands in the fingerprint region of the IR spectra of 2 and 5 (Figure S25) confirms the changes in the structure and bonding environment that follow from the photoinduced [2 + 2] cycloaddition.
Conclusion
In conclusion, the first isolated example of a complex featuring more than one halogen(I) environment, 2, was characterized in the solid state. Complex 2, which contained [N―I―N]+, O―I―N, and [O―I―O]− groups, was found to undergo conversion in solution to its corresponding bis(OIN) complex, 1a, as confirmed by solution NMR studies of redissolved crystalline samples of 2, as well as by comparison to the cation of 2 ([I(4-stypy)2]+) separately prepared as a [PF6]- salt, 3. Given the favorable solid-state orientation of the 4-stypy substituents in 2, subsequent UV irradiation studies were performed on crystalline samples of 2. These studies resulted in the single-crystal-to-single-crystal transformation of the discrete salt 2 to the 1D polymeric complex 5, via the [2 + 2] cycloaddition of the alkenyl groups of the 4-stypy substituents, making it the first nondestructive photochemical conversion of a halogen(I) species. The solid-state structure of 5 was found to retain all the iodine(I) moieties found in 2, with the mechanical strain of the newly formed cyclobutane rings predominantly borne by the pyridyl groups coordinating to the O―I―N moieties. These results further expand the toolbox of methodologies available in the field of halogen bonding, particularly toward constructing polymeric networks without the need to incorporate multi-dentate ligands that are traditionally used to construct coordination networks (cf. XOFs). This utility has potential toward synthesizing targeted functional materials, with other photoactive systems currently being investigated.
Supporting Information
The authors have cited additional references within the Supporting Information.[100-104]
Acknowledgements
Dr. Aleksei Emelianov is thanked for guidance on the use of the Raman spectrometers. Dr. Pasi Myllyperkiö is thanked for assistance in performing the UV irradiation experiments. The authors gratefully acknowledge the Magnus Ehrnrooth Foundation (JSW) and the Research Council of Finland (formerly the Academy of Finland) grant numbers 356187 (J.S.W.) and 336456 (A.M.) for funding. [Correction added on 8 July 2025, after first online publication: FinELib 2023 funding statement has been added.]
Open access publishing facilitated by Jyvaskylan yliopisto, as part of the Wiley - FinELib agreement.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.