Intriguing Reactivity of a 1,2-Dihydrodialumane Towards Organic Azides – From a Terminal Diazido–Dialumane to Pendulum-Clock-Like Azide Bridging
Dedicated to the 90th birthday of Professor Herbert W. Roesky and the 25th anniversary of his [nacnacAl(I)]
Graphical Abstract
The reaction of a dihydro-dialane supported by a hybrid ligand (DNI) with organic azides yields structurally diverse aluminium species, including the first example of low oxidation state aluminium azide complex, a diazido-dialane, along with an aluminium-tetrazole, and azide alumination. Spectroscopic studies reveal dynamic behaviour in solution, featuring a pendulum-clock-like motion of the benzyl azide and Nα oscillation within the complex.
Abstract
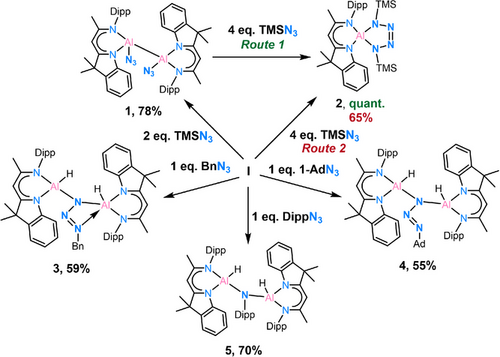
The reactivity of dihydro-dialane with organic azides is described. Treatment of the hybrid ligand-based dialane [(DNI{H}Al)2] (I) (DNI = [3,3-dimethyl-2-2-methyl-2-(2,6-diisopropylaniline)ethenyl]-3H-indolenine) with TMSN3 (TMS = trimethylsilyl) at room temperature gives the first diazido-dialane [(DNI{N3}Al)2] (1). The transformation from here to a more stable aluminium-tetrazole [DNIAl(NTMS)2N2] (2) is established. The reaction of other RN3 gives [(DNI{H}Al)2(κ2-N3R)] (R = Benzyl in 3 and 1-Adamantyl in 4) with the azide in a μ-bridging position between two aluminium atoms. Using 1H NOESY/EXSY NMR spectroscopy, a positional exchange of the two TMS groups (2) via rotation of the tetrazole unit is observed. In contrast, compound 3 exhibits a pendulum-clock-like dynamic, with Nα oscillating between the two aluminium atoms in solution. The reaction of dialane I with DippN3 (Dipp = 2,6-iPr2-C6H3) gives the dialuminium amine [{DNI(H)Al}2(μ-NDipp)] (5).
Introduction
Main-group chemistry, involving central elements in atypical electronic configurations – such as varied oxidation states – demonstrates great promise in activating strong chemical bonds and advancing catalytic processes.[1-6] While redox-type oxidative addition and reductive elimination are typically associated with transition metals, such reactivity has increasingly been observed in main-group systems. Aluminium, the most abundant metal in the Earth's crust, exemplifies this potential. In the solid state, trivalent aluminium (Al3+) plays a key role in materials like zeolites, aluminas, and oxide frameworks, where it enables catalysis, ion exchange, and gas separation.[7-10] Aluminium chemistry has recently expanded beyond its conventional +III state, with growing interest in +I oxidation state species.[11-15] These include neutral aluminylenes, first discovered in 1991,[16] and aluminyl anions established in 2018,[17] both of which, along with their successors and other low-valent aluminium derivatives, have emerged as true powerhouses in selectively activating and transforming even the thermodynamically strongest bonds.[13, 18-24]
Neutral complexes of the type R2AlAlR2, which possess Al—Al single bonds [commonly referred to as dialumanes or dialane(4)], represent another remarkable class of low-valent aluminium complexes where both aluminium atoms are in the +II oxidation state. Isolating and stabilizing these complexes is rather challenging due to the small Al─Al bond energy, the electropositive nature of Al, and the inherent Lewis acidity of these species with formal six valence electrons.[13] Indeed, the isolation of these complexes was predicted to be inaccessible based on calculations for Al2H4, stating that complexes of this type will generally decompose through disproportionation.[25] This assumption was debunked with the first dialumane synthesis of [{(Me3Si)2CH}2Al]2, which was structurally characterized. Its isolation was enabled by the support of kinetically stabilizing and sterically demanding substituents (Scheme 1a).[26] Since then, this field has rapidly expanded.[13, 27-30] Consequently, various 1,2-dihalodialumanes,[31-34] first examples of unsymmetrically functionalized dialumanes,[35, 36] and 1,2-dihydrodialanes have been synthesized and thoroughly characterized in recent years.[13, 22, 37-40] Remarkably, even the parent 1,2-dihydrodialane Al2H4 can be tamed through stabilization by N-heterocyclic carbenes (Scheme 1b).[37] Despite these significant developments in the field, the reactivity of dialumanes remains relatively underexplored due to their propensity for disproportionation into corresponding Al(I) and Al(III) complexes (Scheme 1c). This includes 1,2-dihydrodialanes, which tend to disproportionate into respective dihydroalanes and aluminylene species. These reactions were found to be in equilibrium, suggesting facile reductive elimination at the Al─H bonds and raising expectations for catalytic applications of these complexes in the foreseeable future.[4, 22, 37, 39]
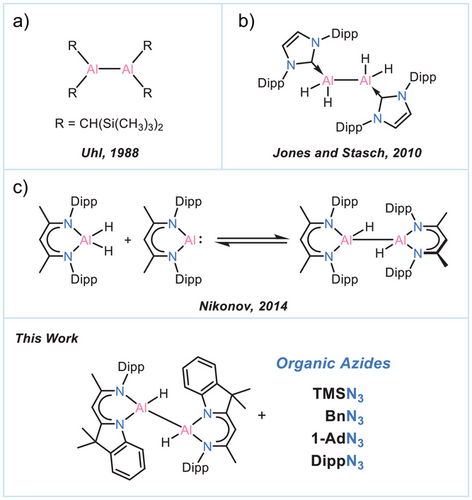
We have recently reported the straightforward synthesis of 1,2-dihydrodialane [(DNI{H}Al)2] (I), supported by a β-diketiminato-indole-hybrid ligand (DNI),[41] and revealed its propensity to cleave the CAr(sp2)─H bond.[42] Given the scarce reports on the selective reactivity of 1,2-dihydrodialanes (vide supra), we herein present the diverse reactivity of I towards a series of organic azides. Sterically encumbered azides have ever since been in the focus of low-valent aluminium chemistry.[43-45] In this paper we present the wide product range from a unique and highly reactive terminal diazido-dialane to pendulum-clock-like bridged aluminium azide species. Accordingly, this study represents the first investigation of 1,2-dihydrodialanes' reactivity towards organic azides. Our findings highlight new reaction pathways that are distinctly different from those observed in the limited reports on the reactions of dihalo- and diamido-substituted dialumanes.[34, 46]
Results and Discussion
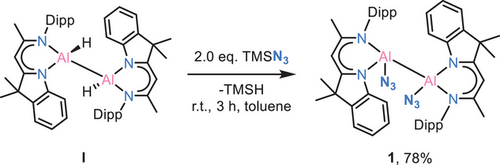
The reaction of dialane [(DNI{H}Al)2] (I) with 2.0 eq. TMSN3 in toluene at room temperature gives the diazido-dialane complex [(DNI{N3}Al)2] (1) with a good yield of 78%. Rapid crystal formation was observed upon cooling the solution to −30°C (Scheme 2).
Complex 1 crystallizes in the triclinic space group P with two toluene molecules in the asymmetric unit. The molecular structure reveals the maintenance of the Al─Al bond with each Al(II) atom coordinated by an azide group (Figure 1). The Al─Al bond length of 2.6008(12) Å remains almost identical to that of the dialane [(DNI{H}Al)2] (I) starting material (2.6007(6) Å). The Al─N bond lengths to the azide group are 1.8669(18) Å (Al1─N5) and 1.8686(18) Å (Al2─N8), respectively. Accordingly, they are slightly longer than in the previously reported Al(III)−N3 complex, for example, [(DippBDIAl{N3}N{μ-Si(N3)tBu})2] by Roesky[47] and [TeriPr8Al{N3}{N(SiMe3)2}] by Power.[44] These differences can be attributed to the slightly smaller radius of Al(III) compared to Al(II). In 1 the two azide groups point in the same direction. Their structural parameters are N5─N6 1.203(2) Å, N6─N7 1.150(2) Å, N8─N9 1.210(2), N9─N10 1.142(2), and they adopt a slightly nonlinear arrangement of Nα─Nβ─Nγ with 175.91(19)° for N5─N6─N7 and 176.2(2)° for N8─N9─N10, respectively.
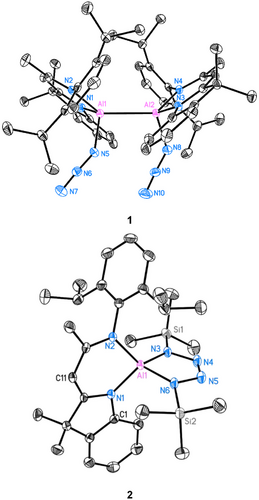
The dislocation of the two Al atoms from their C3N2 mean planes are minute with d(Al···C3N2) = 0.039(2) Å for Al1 and 0.048(2) Å for Al2. This is consistent with the slight folding angle of the ligand C3N2 mean plane [N1─C10─C11/N2─C12─C11: 4.1(4)°, N3─C35─C36/N4─C37─C36: 5.3(3)°].
Density functional theory (DFT) calculations at the B3LYP/6–31+G(d) level of theory were carried out using crystallographic coordinates to investigate the bonding in 1. The calculated energies of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are presented in the Supporting Information (see Figure S35). The HOMO, and similarly the HOMO-2, is primarily localized on the Al─Al σ-bond, while significant contributions to the molecular orbitals from the azide ligands are noted between HOMO-3 and HOMO-5. This highlights the electronic interplay between the aluminium core and the azide groups.
The Al─Al interaction is characterized by a non-nuclear attractor (NNA), a local maximum in the electron density that does not coincide with any nucleus, as first predicted by Gatti and Bader[48] later experimentally verified by Platts et al.[49] Subsequently, it was observed in our previous work[42] and reported by others.[50-55] QTAIM analysis[56] assigns to the NNA an electron density of 0.06 e−/bohr3 and a charge of −0.909 e−, indicating localized electron accumulation in the interatomic region (Figure 2 and Table S8 in SI). This represents the first observation of an NNA in an aluminium azide complex. Additionally, the Wiberg bond index (WBI) of 0.91 further supports a single-bond framework, highlighting the shared electronic interaction facilitated by the NNA. The Al─N bond lengths to the azide units reflect moderate covalency. The QTAIM analysis reveals slightly elevated charge densities at the Al─N bond critical points (BCPs) compared to other Al─N ligand bonds, consistent with the electron-withdrawing inductive effect of the azide groups. Notably, natural bond orbital (NBO) calculations (see Figure S36 and Table S10 in the Supporting Information) indicate partial charge transfer from the Al atoms to the azide moieties, highlighting their role as strong electron acceptors. This effect is further supported by an increase in the partial charges on Al1 and Al2 compared to the parent dialane I (2.102 and 2.129 e−, respectively). The observed Δq increases, Δq(Al1) = +0.047 and Δq(Al2) = +0.026, suggest that the azide groups exert a stronger electron-withdrawing inductive effect than the hydrogen substituents in dialane I.
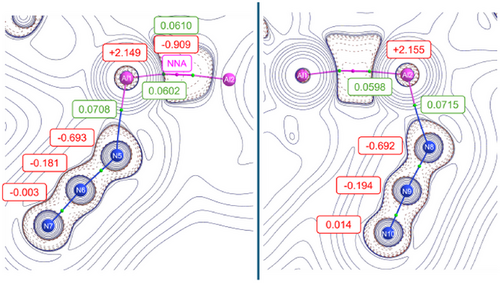
The azide groups themselves exhibit distinct bonding features. The N─N electron density at the BCPs ranges from 0.465 to 0.553 e−/bohr3, showcasing partial delocalization, with stronger covalent character of the terminal N─N bonds. Ellipticity values derived from QTAIM indicate a mixed bonding character, with the terminal N─N bonds (Table S8) displaying lower ellipticity compared to the central N─N bond, suggesting a more pronounced π-contribution in the former, pointing towards triple bond behaviour. The Wiberg bond indices for the azide N─N bonds, averaging to 1.62 for the central bonds and 2.24 for the terminal bonds (see Table S9 in SI), supporting these trends with the terminal bonds indicating stronger multiple-bond character.
Complex 1 remains stable in the solid-state for approximately one week at −30°C. However, in solution, it gradually decomposes into unidentified products under ambient conditions, leading to poorly resolved ¹H NMR spectra despite multiple attempts. Both 1H and 13C NMR spectroscopy indicate the presence of multiple species in solution. Additionally, 1H DOSY NMR experiments[57-61] further support the decomposition of complex 1 (Figures S2–S4 in SI). Attempts to determine the molecular weight revealed that the decomposed products have lower molecular weights than the parent diazido-dialane (856.5 gmol−1); however, an unambiguous structural assignment was not possible. Notably, the decomposition process accelerates significantly at elevated temperatures.
To the best of our knowledge, 1 is the first reported example of a diazido-dialane, and its reactivity remains unexplored. In here, however, it was serendipitously discovered that the addition of an excess of TMSN3 to 1 at −30°C for one week (Route 1, Scheme 3) results in the formation of a thermally stable aluminium-containing tetrazole complex [DNIAl(NTMS)2N2] (2) in quantitative yield. Alternatively, the addition of 4.0 eq. TMSN3 to the starting dialane material I (Route 2, Scheme 3) followed by warming to 70°C for 18 h, consistently yields the identical yellow crystalline solid of 2 with a yield of 65%. It is worthy to note that even when 2.0 eq. of TMSN3 were employed, only 2 can be isolated. Its single crystal X-ray structure is depicted in Figure 1. Complex 2 crystallised in space group C2/c with one moelcule and a half toluene molecules in the asymmetric unit. The molecular structure reveals a distorted tetrahedral coordination geometry at the aluminium atom. It exhibits notable dislocation of d(Al···C3N2) = 0.5363(17) Å from the ligand's C3N2 mean plane. The five-membered tetrazole ring, however, is planar (d(Al···(NTMS)2N2) = 0.012(2) Å). The Al1−N3 (1.8260(13) Å) and Al1−N6 (1.8312(13) Å) distances are similar to those reported for [DippBDIAl(NTMS)2N2][62] (d(Al−N): 1.8152(15) Å, 1.851(2) Å).
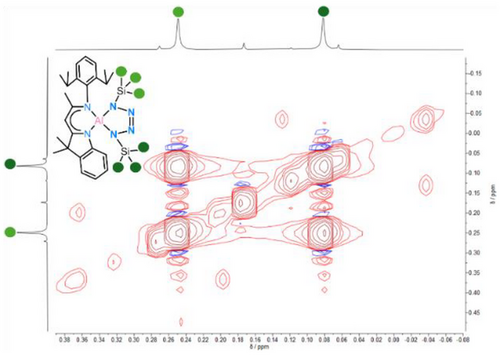
The 1H NMR spectroscopic signal of backbone proton H11 (δ = 5.08 ppm) is slightly downfield shifted compared to the starting material I (δ = 5.01 ppm). The protons of the trimethylsilyl group display two separate signals at δ = 0.25 and 0.08 ppm. The 27Al NMR spectrum shows a single signal at δ = 77.49 ppm while the 1H,29Si HMBC NMR spectrum shows two different signals at δ = 2.5 and −1.6 ppm, respectively. Especially the 1H EXSY NMR spectrum reveals exchange signals between the two TMS methyl groups (Figure 3). This observation suggests dynamic behaviour of the tetrazole moiety in solution, indicating a position change of the two TMS groups via rotation of the tetrazole unit while the ligand geometry is preserved.
Even given the existing reports of aluminium-tetrazoles synthesized by Roesky,[47, 62] Aldridge,[63] and Power,[44] the synthesis of aluminium-tetrazoles via dihydrodialane or diazido-dialane was previously unknown. Based on the recent and previously reported examples, we propose that the final step in forming the tetrazole complex 2 involves a [2+3] cycloaddition between an aluminium-imide intermediate [DNIAl═NTMS] (9) and an additional azide molecule. The aluminium-imide intermediate is suggested to be formed through the reaction of [DNIAl:] (8) with an azide, accompanied by the release of dinitrogen gas. This leads to the critical point that generating monomeric [DNIAl:] (8) is clearly the key step in this reaction. Accordingly, we propose a mechanism to explain this process, shown in the Figure 4. It begins with the azidation of dialane I with 2.0 eq. of TMSN3, yielding diazido-dialane 1. NMR spectroscopy suggests the decomposition of 1 in solution, potentially involving the release of N2 gas from the parent diazido-dialane. This transformation most likely proceeds, similar to Staudinger-type reaction, via the formation of the Al2N2 four-membered ring complex [(DNI)Al(μ-η¹:η¹-N2)Al(DNI)] (6). In this process, the liberation of diatomic nitrogen reduces Al(II), leading to the formation of a transient dialumene species, [(DNI)Al═Al(DNI)] (7), accompanied by the release of another equivalent of N2. The comparison of experimental conditions in Route 1 and Route 2 confirms that this sequence of reactions is endothermic. Notably, the dissociation of dialumene to the monomeric aluminium(I) species has previously been firmly recognized and characterized as an endothermic process.[39, 46, 64, 65] Drawing from studies on aluminium-tetrazole complexes,[44, 47, 62, 63] the subsequent reaction pathway involves the formation of an iminodialane intermediate [(DNI)Al═N(TMS)] (9), undergoing a [2+3] cycloaddition and ultimately resulting in the formation of a five-membered aluminium-tetrazole ring. This sequence rationalizes the observed experimental reactivity and the corresponding products.
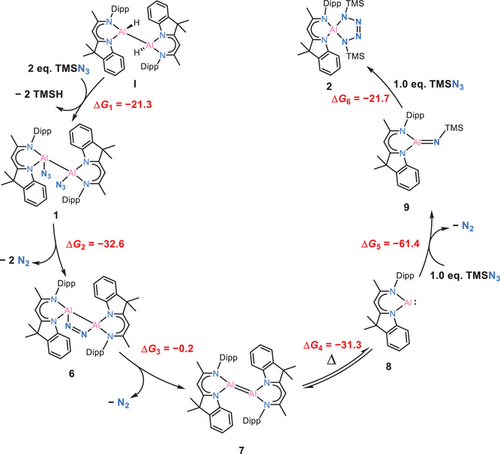
To examine the proposed mechanism for the formation of the tetrazole compound 2, a series of DFT calculations were conducted to evaluate the thermodynamic feasibility of each elementary step (Figure 4 and Supporting Information). The transformation begins with the reaction of dialane I [(DNI{H}Al)2] with two equivalents of TMSN3, forming the diazido-dialane 1 and releasing two equivalents of TMSH (ΔG1 = −21.3 kcal mol−1). Subsequently, 1 decomposes via extrusion of dinitrogen to form the Al2N2 four-membered ring compound 6 (ΔG2 = −32.6 kcal mol−1), a structural motif reminiscent of an intermediate proposed in Staudinger-type reactivity. Further loss of dinitrogen from 6 affords the dialumene 7 (ΔG3 = −0.2 kcal mol−1), which then dissociates into two monomeric aluminium(I) [DNIAl:] 8 (ΔG4 = −31.3 kcal mol−1). Although dissociation of dialumenes into monomeric Al(I) species is typically considered endergonic due to the associated bond dissociation energy, the process is thermodynamically favourable in this case. This can be rationalized by the high reactivity and pronounced instability of 7, which likely destabilizes the dimer relative to the monomers in our system. The highly reactive aluminium(I) species 8 then reacts with TMSN3 to afford the iminoalane 9, accompanied by the release of N2 (ΔG5 = −61.4 kcal mol−1). A second equivalent of TMSN3 converts 9 to the final tetrazole product 2 (ΔG6 = −21.7 kcal mol−1). All individual steps are exergonic, supporting the thermodynamic plausibility of the proposed multistep mechanism.
The observed reactivity encouraged further investigation with other organic azides. Thus, BnN3, 1-AdN3, and DippN3 were separately reacted with dialane I in 1:1 ratio at room temperature. Complex [(DNI{H}Al)2(κ2-N3Bn)] (3) was obtained in the reaction with BnN3 in toluene as yellow crystalline solid in good yield (Scheme 3, 59%). The single crystal X-ray structure determination unambiguously reveals a bridging organo-azido group and a five-coordinated aluminium centre (Al2) exhibiting a κ2 planar coordinated geometry [d(Al···N3,azide) = 0.0077(4) Å] (see Figure 5). The Al─N bond lengths to the five-coordinated aluminium atom Al2 of d(Al2─N3) = 1.9492(14) Å and d(Al2─N4) = 1.9405(14) Å are longer than d(Al1─N1) = 1.9061(15) Å and d(Al1─N2) = 1.8877(14) Å, which is attributable to the higher coordination number. A significantly longer additional δ-donor bond N7→Al2 is formed at 2.1633(15) Å.
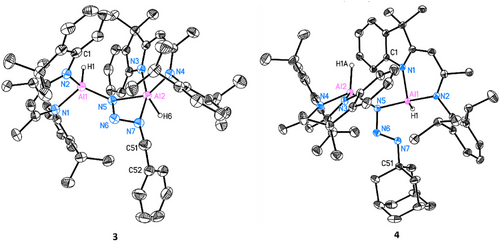
The reaction of dialane I with 1-AdN3 in toluene yielded the crystalline complex [(DNI{H}Al)2(μ-N3Ad)] (4, 54%, Scheme 3). The molecular structure, as shown in Figure 5 reveals the presence of a bridging azido group. The orientation of N7 towards Al1 results in a much longer Al1···N7 distance of 2.3347(12) Å than in 3, suggesting an even weaker interaction. The bending angle of N5─N6─N7 (109.42(10)°) is slightly larger than in 3 (108.17(13)°). Additionally, the N─N distances within the azido group [N5─N6 1.3565(15), N6─N7 1.2739(15) Å] indicate less electron delocalization compared to complex 3.
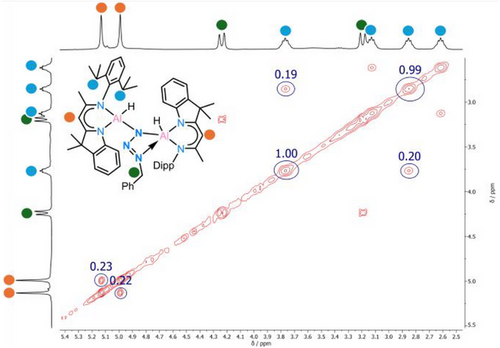
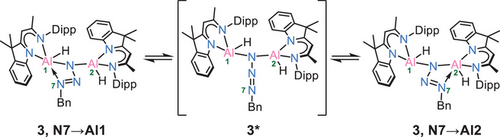
In the solid-state, 3 remains stable for at least one month when stored at −30°C. Similarly, in toluene solution, 3 exhibits a remarkable thermal stability, with neither N2 loss nor significant changes in the ¹H NMR spectrum observed, unless heated to 120°C for 1 h. At room temperature additional insights into the dynamic of 3 in solution comes from NMR analysis. Two distinct backbone signals are visible at 5.13 and 4.99 ppm, respectively. The 1H-EXSY spectrum recorded at 298 K (Figure 6) reveals exchange signals involving the two backbone resonances and several other protons, including those of the Bn─CH2 group (highlighted in green). This indicates that N7 is not rigidly coordinated to either of the aluminium atoms (Al1, Al2) but rather undergoes continuous oscillation between them via 3*, with an estimated exchange rate of ∼4 s⁻¹. This dynamic behaviour resembles a pendulum-like motion of the N3Bn group, similar to that observed from the tetrazole moiety in 2 (Scheme 4).
Attempts to obtain satisfactory 1H, 13C, and 2D NMR spectra of 4 at 298 K were unsuccessful, likely due to its instability in solution. Consequently, NMR measurements were attempted at 243 K. However, despite multiple attempts, a satisfactory spectral analysis could not be achieved either. However, DOSY NMR spectroscopy with adamantane as an internal reference in tol-d8 at 243 K was instructive. The diffusion coefficients of the selected singlets at δ = 5.35, 5.22, 4.91, 4.80, and 4.70 ppm in the backbone region showed only minimal differences, ranging from 1.646 to 1.665 × 10−10 m2. This indicates that the species in solution are likely not undergoing significant structural changes or aggregation. However, the molar mass calculated from the diffusion coefficients (MWcalc = 883 g mol−1) is lower than the expected molar mass of 4 (MWdet = 952 g mol−1). From that, we propose that this outcome may result from the gradual release of nitrogen gas in solution, leading ultimately to the formation of [(DNI{H}Al)2(μ-NAd)]. To facilitate azide decomposition, the solution was heated to 60°C for 1 h, which resulted in a significant darkening of its colour. Unfortunately, attempts to crystallize and isolate the expected dialuminium amine [(DNI{H}Al)2(μ-NAd)] as the product from nitrogen gas liberation were unsuccessful.
Building on this structural information, the 1H-NOESY NMR spectrum of complex 4 shows no clear exchange signals, indicating that the pendulum-clock dynamic observed in 2 and 3 is absent in 4. This confirms that the bridging azide in complex 4 exists in a relatively “free” state in solution, with the Nα atom not directly coordinating to either aluminium atom.
The reaction between dialane I and the more sterically demanding DippN3 azide was anticipated to form the κ2- or μ-N3R (R = Dipp) structure like in 3 and 4. Unexpectedly, a significant release of gas was observed during the reaction, enabling the formation of a dialuminium amine product impossible to isolate from the reaction with Ad─N3. Crystals suitable for SC-XRD experiments are obtained by leaving the benzene solution at room temperature for one day. The structure confirms the N2 loss during the reaction, resulting in a N─Dipp bridging moiety between the two aluminium atoms (Figure 7).
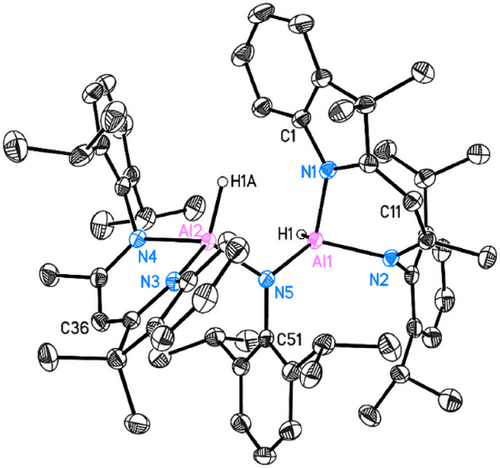
5 crystallises in the monoclinic space group P21/n with one molecule and disordered lattice solvent in the asymmetric unit. The structure shows a significant butterfly folding of the ligand's C3N2 mean planes (N1─C10─C11/N2─C12─C11: 21.27(12), N3─C35─C36/N4─C37─C36: 18.5(2)°), resulting in a remarkable dislocation of aluminium from the ligand's C3N2 mean plane [d(Al1···C3N2) = 0.7668(14) Å; d(Al2···C3N2) = 0.7150(14) Å].
The 1H NMR spectrum shows two singlet signals at δ = 5.33 and 4.82 ppm, respectively, assigned to the ligand's backbone. (See Supporting Information) The Dipp─CH protons at both ligands show four signals at δ = 3.89, 2.59, 2.45, and 2.23 ppm, respectively. The 1H-NOESY NMR spectrum of 5 shows cross-peaks between the Dipp─CH protons of two different groups: one belonging to the bridging Dipp ligand and the other attached to the DNI ligand. This observation is corroborated by the solid-state structure, which reveals a spatial distance of 2.113 Å between H45 and H57, close enough to generate cross-peaks in the 1H-NOESY spectrum. Consequently, the protons of the bridging Dipp group are unambiguously assigned to two distinct signals at δ = 4.31 and 3.60 ppm. These findings confirm that the molecule retains the solid-state structure in solution.
Conclusion
In summary, we have investigated the reactivity of hybrid-ligand based (DNI) dialane [(DNI{N3}Al)2] (I) with different organo-azides, containing various bulky substitutes. The first low oxidation state aluminium azide complex, diazido-dialane [(DNI{N3}Al)2] (1), was successfully isolated and characterized. Notably, complex 1 was identified as an isolable intermediate in the reaction of dialane with TMSN3 at elevated temperature, ultimately yielding the stable tetrazole complex [DNIAl(NTMS)2N2] (2), most probably via (DNI)Al═Al(DNI) and (DNI)Al═N(TMS). The reaction of dialane with BnN3 and 1-AdN3 both led to the formation of bridged azido-dialane complex 3 and 4 ([(DNI{H}Al)2(κ2-N3R)] (R = Bn in 3 and 1-Ad in 4). Detailed 1H EXSY NMR spectroscopy reveals for 2 a positional exchange of the two TMS groups through the rotation of the tetrazole unit and 3 exhibits pendulum-clock-like dynamics in solution, with exchange rate successfully determined to be 4 s−1. On the other hand, the reaction of dialane with DippN3 yielded the dialuminium amine product [{DNI(H)Al}2(μ-NDipp)] (5). Hence, this work expands the understanding of the reactivity of dialane with organic azides considerably.
Experimental
General Procedures
All manipulations that require processing under inert conditions were carried out under an argon atmosphere using standard Schlenk techniques or an MBraun Glovebox. Toluene was distilled from sodium. Toluene-d8, benzene and benzene-d6 were distilled from potassium. n-Hexane was distilled from Na/K alloy. All solvents were routinely degassed three times using the freeze-pump-thaw method and stored in a glove box over molecular sieve. The discussed dialane was synthesised according to a literature procedure.[42] TMSN3 and 1-AdN3 were purchased from Sigma Aldridge. BnN3 was purchased from ABCR. DippN3 was synthesised according to a literature procedure.[66] 1-AdN3 was purified by stirring a toluene solution of 1-AdN3 over molecular sieves, followed by filtration and vacuum drying. Elemental analysis was performed on an Elementar Vario EL3 by the Analytisches Labor, Institut für Anorganische Chemie, Universität Göttingen. Mass spectra were measured by the Zentrale Analytik within the Faculty of Chemistry, Universität Göttingen applying a Liquid Injection Field Desorption Ionisation-technique on a JEOL accuTOF instrument with an inert-sample application setup under argon atmosphere. Fluorescence analyses were conducted using a FluoroMax-4 spectrometer from HORIBA Jobin Yvon. The quantum yield was determined with the quanta-φ integrating sphere. At the host computer, the software FluorEssence v3.0 and OriginPro 8.5 G were used to record the information of the fluorescence spectra and analyse the data in the graph. The 1H, 13C, 15N, and 2D NMR spectroscopic data were recorded on a Bruker Avance III 300 MHz, a Bruker Avance III HD 400 MHz, a Bruker Avance III HD 500 MHz, and a Bruker Avance NEO 600 MHz instrument.
Crystallographic Details
Crystals were selected from a Schlenk flask under an argon atmosphere, then put into a droplet of perfluorinated polyether oil on a microscope slide, and immediately shock cooled using the X-TEMP2 device.[67, 68] The diffraction data were collected using Mo Kα radiation and a Bruker Photon III C7 Detector. The data were integrated with SAINT.[69] A multi-scan absorption correction was applied using SADABS[70] or TWINABS.[71] The structures were solved by SHELXT[72] and refined on F2 using SHELXL.[73] in the graphical user interface ShelXle.[74] All hydrogen atoms bond to carbon atoms were placed according to geometrical criteria and refined with a riding model.
Computational Details
Single-molecule calculations were performed with the use of Gaussian16 software[75] at the B3LYP/6–31+G(d) level of theory, which offers a balance between accuracy and computational cost. Coordinates were taken from crystallographic data and were kept frozen for further calculations. Such obtained wave function was further used to perform QTAIM partitioning of electron density with the use of AIMAll software.[76] Natural bonding analysis of compound 1 was performed with the natural bond orbital[77] (NBO 3.1) partitioning scheme implemented in the Gaussian16. Natural charges and Wiberg bond indices[78] (WBI) were obtained directly from NBO analysis. To evaluate the free energy changes associated with the proposed mechanism, initial geometry optimizations were performed at the HF/6–31G(d) level in toluene, modelled with the polarizable continuum model (PCM). These optimized geometries served as the starting point for subsequent refinements that incorporated electron correlation effects using the B3LYP functional with the same basis set. More detailed information on these free energy calculations is provided in the Supporting Information.
Supporting Information
Experimental and characterization details for all new complexes, including spectroscopic, crystallographic, and theoretical data are available in the Supporting Information.
Acknowledgements
The authors thank David M.J. Krengel for synthesizing DippN3. A.K. and R.F.L. gratefully acknowledge Scientific Computer Cluster at the Faculty of Chemistry, University of Göttingen for providing computational resources.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.