Stable Yet Strongly Lewis-Acidic Anions Enabling Cooperative Catalysis with Cationic Transition-Metal Complexes
Ryo Mandai
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Iwasaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, Japan
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Kyoko Nozaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
E-mail: [email protected]; [email protected]
Search for more papers by this authorRyo Mandai
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Iwasaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, Japan
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Kyoko Nozaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
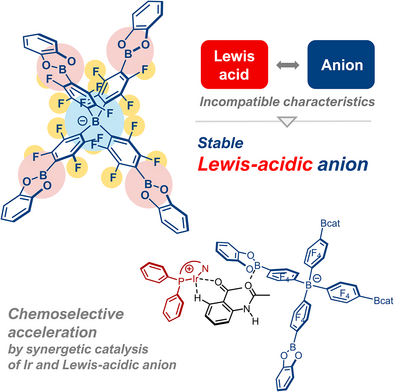
A stable tetraarylborate anion, BBcat, possessing strong Lewis acidity was synthesized. BBcat serves as a weakly coordinating counteranion for the cationic transition-metal complex, and its Ir complex exhibited up to 8.2-fold higher reaction rate than the common counteranion BArF in the hydrogen isotope exchange of 1,2-disubstituted arenes by cooperative catalysis of the cationic transition-metal complex and the Lewis-acidic counteranion.
Abstract
Ionic transition-metal complexes play a crucial role as catalysts in organic transformations. Their counteranions often stand aside from the catalytic cycle or occasionally participate in the catalytic cycle as a Brønsted base due to their nucleophilic character. Herein, we developed a stable yet Lewis-acidic anion, BBcat, based on tetrakis(pentafluorophenyl)borate and featuring Lewis-acidic catechol borane moieties and applied it to transition-metal catalysis to recognize Lewis-basic substrates. Upon admixture with Bu4N–BBcat, 31P NMR chemical shift of O═PEt3 significantly lower-shifted, implying the strong Lewis acidity of BBcat despite being an anion. In an Ir complex supported by a bidentate phosphine ligand, BBcat resides in the second coordination sphere as a noncoordinating counteranion. Ir/PHOX-BBcat exhibited an 8.2-fold higher reaction rate than Ir/PHOX-BArF consisting of the non-coordinating anion in the hydrogen isotope exchange of acetophenone derivatives bearing additional Lewis-basic functionalities. The acceleration effect depends on the steric hindrance and basicity of the additional Lewis-basic sites, located at remote positions to the C─H bond to be deuterated. These results clearly indicate that the interaction of BBcat with the Lewis-basic sites plays a crucial role, facilitating cooperative catalysis of the cationic transition-metal center and the counteranion.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202503322-sup-001-SuppMat.pdf8.7 MB | Supporting Information |
| anie202503322-sup-0002-cif.zip249.1 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Representative examples for multifunctional catalysts bearing recognition sites in their second coordination sphere: R. Breslow, X. Zhang, Y. Huang, J. Am. Chem. Soc. 1997, 119, 4535–4536.
- 2S. Das, C. D. Incarvito, R. H. Crabtree, G. W. Brudvig, Science 2006, 312, 1941–1943.
- 3T. Šmejkal, B. Breit, Angew. Chem. Int. Ed. 2008, 47, 311–315.
- 4P. Dydio, W. I. Dzik, M. Lutz, B. de Bruin, J. N. H. Reek, Angew. Chem. Int. Ed. 2011, 50, 396–400.
- 5K. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712–717.
- 6H. L. Li, Y. Kuninobu, M. Kanai, Angew. Chem. Int. Ed. 2017, 56, 1495–1499.
- 7L. Yang, N. Uemura, Y. Nakao, J. Am. Chem. Soc. 2019, 141, 7972–7979.
- 8M. E. Hoque, R. Bisht, C. Haldar, B. Chattopadhyay, J. Am. Chem. Soc. 2017, 139, 7745–7748.
- 9H. J. Davis, M. Mihai, R. J. Phipps, J. Am. Chem. Soc. 2016, 138, 12759–12762.
- 10D. M. Beagan, C. Rivera, N. K. Szymczak, J. Am. Chem. Soc. 2024, 146, 12375–12385.
- 11Selected examples: M. F. Perutz, G. Fermi, B. Luisi, B. Shaanan, R. C. Liddington, Acc. Chem. Res. 1987, 20, 309–321.
- 12T. L. Poulos, B. C. Finzel, A. J. Howard, J. Mol. Bid. 1987, 195, 687–700.
- 13D. W. Christianson, J. D. Cox, Annu. Rev. Biochem. 1999, 68, 33–57.
- 14S. P. de Visser, Chem. Eur. J. 2020, 26, 5308–5327.
- 15B. M. Hoffman, D. Lukoyanov, Z.-Y. Yang, D. R. Dean, L. C. Seefeldt, Chem. Rev. 2014, 114, 4041–4062.
- 16D. Zuccaccia, A. D. Zotto, W. Baratta, Coord. Chem. Rev. 2019, 396, 103–116.
- 17L. Biasiolo, M. Trinchillo, P. Belanzoni, L. Belpassi, V. Busico, G. Ciancaleoni, A. D'Amora, A. Macchioni, F. Tarantelli, D. Zuccaccia, Chem. Eur. J. 2014, 20, 14594–14598.
- 18M. Trinchillo, P. Belanzoni, L. Belpassi, L. Biasiolo, V. Busico, A. D'Amora, L. D'Amore, A. D. Zotto, F. Tarantelli, A. Tuzi, D. Zuccaccia, Organometallics 2016, 35, 641–654.
- 19G. Ciancaleoni, L. Belpassi, D. Zuccaccia, F. Tarantelli, P. Belanzoni, ACS Catal. 2015, 5, 803–814.
- 20L. Biasiolo, A. L. Zotto, D. Zuccaccia, Organometallics 2015, 34, 1759–1765.
- 21L. D'Amore, G. Ciancaleoni, L. Belpassi, F. Tarantelli, D. Zuccaccia, P. Belanzoni, Organometallics 2017, 36, 2364–2376.
- 22Representative examples of counteranion-directed asymmetric transition metal catalysis: D. B. Llewellyn, D. Adamson, B. A. Arndtsen, Org. Lett. 2000, 2, 4165–4168.
- 23G. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496–499.
- 24S. Mukherjee, B. List, J. Am. Chem. Soc. 2007, 129, 11336–11337.
- 25C. Li, C. Wang, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 2008, 130, 14450–14451.
- 26M. Barbazanges, M. Augé, M. Moussa, H. Amouri, C. Aubert, C. Desmarets, L. Fensterbank, V. Gandon, M. Malacria, C. Ollivier, Chem. Eur. J. 2011, 17, 13789–13794.
- 27S. Liao, B. List, Angew. Chem. Int. Ed. 2010, 49, 628–631.
- 28S. Satake, T. Kurihara, K. Nishikawa, T. Mochizuki, M. Hatano, K. Ishihara, T. Yoshino, S. Matsunaga, Nat. Catal. 2018, 1, 585–591.
- 29A. G. Massey, A. J. Park, J. Organomet. Chem. 1964, 2, 245–250.
- 30H. Nishida, N. Takada, M. Yoshimura, T. Sonoda, H. Kobayashi, Bull. Chem. Soc. Jpn. 1984, 57, 2600–2604.
- 31Pioneering examples for employment of weakly coordinating anions to improve the activity of transition-metal catalysts: M. Brookhart, B. Grant, A. F. Volpe, Organometallics 1992, 11, 3920–3922.
- 32S. P. Smidt, N. Zimmermann, M. Studer, A. Pfaltz, Chem. Eur. J. 2004, 10, 4685–4693.
- 33B. Wüstenberg, A. Pfaltz, Adv. Synth. Catal. 2008, 350, 174–178.
- 34For a review: I. M. Riddlestone, A. Kraft, J. Schaefer, I. Krossing, Angew. Chem. Int. Ed. 2018, 57, 13982–14024.
- 35W. Zierkiewicz, R. Wysokiński, M. Michalczyk, S. Scheiner, ChemPhysChem 2020, 21, 870–877.
- 36S. Scheiner, R. Wysokiński, M. Michalczyk, W. Zierkiewicz, J. Phys. Chem. A 2020, 124, 4998–5006.
- 37S. Scheiner, Chem. Eur. J. 2024, 30, e202402267.
- 38A. Bauzá, A. Frontera, T. J. Mooibroek, Nat. Commun. 2017, 8, 14522.
- 39A. Daolio, A. Pizzi, G. Terraneo, M. Ursini, A. Frontera, G. Resnati, Angew. Chem. Int. Ed. 2021, 60, 14385–14389.
- 40M. Rohdenburg, M. Mayer, M. Grellmann, C. Jenne, T. Borrmann, F. Kleemiss, V. A. Azov, K. R. Asmis, S. Grabowsky, J. Warneke, Angew. Chem. Int. Ed. 2017, 56, 7980–7985.
- 41M. Mayer, V. van Lessen, M. Rohdenburg, G.-L. Hou, Z. Yang, R. M. Exner, E. Aprà, V. A. Azov, S. Grabowsky, S. S. Xantheas, K. R. Asmis, X.-B. Wang, C. Jenne, J. Warneke, Proc. Natl. Acad. Sci. USA 2019, 116, 8167–8172.
- 42M. Mayer, M. Rohdenburg, V. van Lessen, M. C. Nierstenhöfer, E. Aprà, S. Grabowsky, K. R. Asmis, C. Jenne, J. Warneke, Chem. Commun. 2020, 56, 4591–4594.
- 43F. Ebner, H. Wadepohl, L. Greb, J. Am. Chem. Soc. 2019, 141, 18009–18012.
- 44F. Ebner, L. M. Sigmund, L. Greb, Angew. Chem. Int. Ed. 2020, 59, 17118–17124.
- 45L. M. Sigmund, L. Greb, Chem. Sci. 2020, 11, 9611–9616.
- 46L. M. Sigmund, C. Ehlert, M. Enders, J. Graf, G. Gryn'ova, L. Greb, Angew. Chem. Int. Ed. 2021, 60, 15632–15640.
- 47F. Schön, L. M. Sigmund, F. Schneider, D. Hartmann, M. A. Wiebe, I. Manners, L. Greb, Angew. Chem. Int. Ed. 2022, 62, e202202176.
- 48L. M. Sigmund, E. Engels, N. Richert, L. Greb, Chem. Sci. 2022, 13, 11215–11220.
- 49S. J. Kohl, L. M. Sigmund, M. Schmitt, L. Greb, Chem. Sci. 2024, 15, 10803–10809.
- 50Selected examples of cationic Ir-catalyzed HIE; D. Hesk, J. Label. Compd. Radiopharm. 2020, 63, 247–265.
- 51W. J. Kerr, G. J. Knox, L. C. Paterson, J. Label. Compd. Radiopharm. 2020, 63, 281–295.
- 52J. Atzrodt, V. Derdau, W. J. Kerr, M. Reid, Angew. Chem. Int. Ed. 2018, 57, 3022–3047.
- 53J. Atzrodt, V. Derdau, T. Fey, J. Zimmermann, Angew. Chem. Int. Ed. 2007, 46, 7744–7765.
- 54Noteworthy examples for the counteranion effect in HIE: A. R. Kennedy, W. J. Kerr, R. Moira, M. Reid, Org. Biomol. Chem. 2014, 12, 7927–7931.
- 55M. Parmentier, T. Hartung, A. Pfaltz, D. Muri, Chem. Eur. J. 2014, 20, 11496–11504.
- 56A. Mandal, S. Dana, D. Chowdhury, M. Baidya, Chem. Asian J. 2019, 14, 4074–4086.
- 57K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2010, 49, 6169–6173.
- 58B. L. Tóth, A. Monory, O. Egyed, A. Domján, A. Bényei, B. Szathury, Z. Novák, A. Stirling, Chem. Sci. 2021, 12, 5152–5163.
- 59K. Fujiki, J. Ichikawa, H. Kobayashi, A. Sonoda, T. Sonoda, J. Fluor. Chem. 2000, 102, 293–300.
- 60M. Isshiki, H. Kobayahsi, T. Sonoda, The Reports of Institute of Advanced Material Study, Kyushu University 1995, 9, 47–55.
- 61T. Korenaga, T. Kosaki, R. Fukumura, T. Ema, T. Sakai, Org. Lett. 2005, 7, 4915–4917.
- 62Y. P. Budiman, A. Friedrich, U. Radius, T. B. Marder, ChemCatChem 2019, 11, 5387–5396.
- 63R. Takahashi, T. Seo, K. Kubota, H. Ito, ACS Catal. 2021, 11, 14803–14810.
- 64P. Tomaszewski, M. Wiszniewski, J. Serwatowski, K. Woźniak, K. Durka, S. Luliński, Dalton Trans. 2018, 47, 16627–16637.
- 65 Deposition number 2401757 (for Bu4N-BBcat) contains the supplementary crystallographic data for this paper. This data is provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 66U. Mayer, V. Gutmann, W. Gerger, Monatsh. Chem. 1975, 106, 1235–1257.
- 67M. Beckett, G. A. Strickland, Polym. Commun. 1996, 37, 4629–4631.
- 68A. Adamczyk-Woźniak, M. Jakubczyk, A. Sporzyński, G. Żukowska, Inorg. Chem. Commun. 2011, 14, 1753–1755.
- 69J. N. Bentley, C. B. Caputo, Tetrahedron 2019, 75, 31–35.
- 70A. Benton, J. D. Watson, S. M. Mansell, G. M. Rosair, A. J. Welch, J. Organomet. Chem. 2020, 907, 121057.
- 71 The acceptor numbers (ANs) were calculated according to the following formula:[67,68] AN = {(δadduct − δ(1)) /(δ(2) − δ(1))} × 100, where δ(1) and δ(2) are the 31P NMR chemical shifts of O═PEt3 in hexane (41.0 ppm) and in SbCl5 (86.1 ppm).
- 72H. Ruppert, T. Schreyer, M. C. Dietl, M. Rudolph, A. S. K. Hashmi, L. Greb, Z. Anorg. Allg. Chem. 2024, 650, e202400081.
- 73W. M. Haynes, in CRC Handbook of Chemistry and Physics, 95th ed. CRC Press, Boca Raton, USA 2014.
10.1201/b17118 Google Scholar
- 74P. S. Pregosin, E. Martinez-Viviente, P. G. Anil Kumar, Dalton Trans. 2003, 4007–4014.
- 75D. Schott, P. S. Pregosin, A. Albinati, S. Rizzato, Inorg. Chim. Acta 2007, 360, 3203–3212.
- 76E. Martinez-Viviente, P. S. Pregosin, Inorg. Chem. 2003, 42, 2209–2214.
- 77I. Fernandez, E. Martinez-Viviente, P. S. Pregosin, Inorg. Chem. 2005, 44, 5509–5513.
- 78A. Moreno, P. S. Pregosin, L. F. Veiros, A. Albinati, S. Rizzato, Chem. Eur. J. 2008, 14, 5617–5629.
- 79P. G. Anil Kumar, P. S. Pregosin, M. Vallet, G. Bernardinelli, R. F. Jazzar, F. Viton, E. P. Kündig, Organometallics 2004, 23, 5410–5418.
10.1021/om049559o Google Scholar
- 80Selected examples of transition-metal-catalyzed directed-C–H functionalization: S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature 1993, 366, 529–531.
- 81F. Kakiuchi, T. Sato, M. Yamauchi, N. Chatani, S. Murai, Chem. Lett. 1999, 28, 1083–1084.
- 82F. Kakiuchi, T. Sato, T. Tsujimoto, M. Yamauchi, N. Chatani, S. Murai, Chem. Lett. 1998, 27, 1053.
- 83S. Oi, S. Fukita, N. Hirata, N. Watanuki, S. Miyano, Y. Inoue, Org. Lett. 2001, 3, 2579–2581.
- 84M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art, M. Nomura, J. Org. Chem. 1998, 63, 5211–5215.
- 85M. D. K. Boele, G. P. F. Van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries, P. W. N. M. van Leeuwen, J. Am. Chem. Soc. 2002, 124, 1586–1587.
- 86Time-course profile was traced and discussed according to the literature: D. S. Timofeeva, D. M. Lindsay, W. J. Kerr, D. J. Nelson, Catal. Sci. Technol. 2021, 11, 5498–5504.
- 87K. Brak, E. N. Jacobsen, Angew. Chem. Int. Ed. 2013, 52, 534–561.
- 88T. Ooi, K. Maruoka, Angew. Chem. Int. Ed. 2007, 46, 4222–4266.
- 89J. E. Gillespie, A. F. Fanourakis, R. J. Phipps, J. Am. Chem. Soc. 2022, 144, 18195–18211.
- 90Selected examples of ion-paired photocatalysts: E. P. Farney, S. J. Chapman, W. B. Swords, M. D. Torelli, R. J. Hamers, T. P. Yoon, J. Am. Chem. Soc. 2019, 141, 6385–6391.
- 91C. M. Morton, Q. Zhu, H. Ripberger, L. Troian-Gautier, Z. S. D. Toa, R. R. Knowles, E. J. Alexanian, J. Am. Chem. Soc. 2019, 141, 13253–13260.
- 92D. Uraguchi, Y. Kimura, F. Ueoka, T. Ooi, J. Am. Chem. Soc. 2020, 142, 19462–19467.
- 93S. Das, C. Zhu, D. Demirbas, E. Bill, C. K. De, B. List, Science 2023, 379, 494–499.
- 94S. Takizawa, T. Okuyama, S. Yamazaki, K. Sato, H. Masai, T. Iwai, S. Murata, J. Terao, J. Am. Chem. Soc. 2023, 145, 15049–15053.