Dehydrogenative Dual Ammonia Activation and Transfer by an N-Heterocyclic Boryloxy Aluminyl Compound
Graphical Abstract
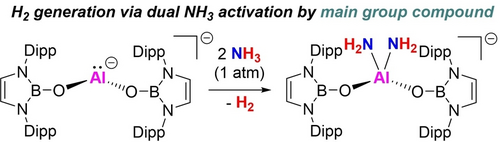
This work showcases the first single-site main-group-mediated dual activation of ammonia (NH3) to hydrogen (H2) using a low-valent aluminyl compound. This transformation is driven by a (coordination/proton shuttling) N−H oxidative addition pathway and selective protonolysis. These findings highlight the potential of main-group complexes in traditionally transition-metal-dominated transformations.
Abstract
The reactivity of an N-heterocyclic boryloxy (NHBO) ligated aluminyl compound has been harnessed for main-group-mediated dehydrogenative dual activation of ammonia. The Al(I) system K[{(HCDippN)2BO}2Al] reacts with excess NH3 to give the Al(III) bis(amide) K[{(HCDippN)2BO}2Al(NH2)2] and in the process generates H2. The initial stage of the reaction proceeds via formal N−H oxidative addition at Al(I) (via a coordination/proton shuttling sequence) to give an aluminium(III) primary amido hydride. Subsequent protonolysis of the strongly hydridic Al−H bond selectively yields the corresponding aluminium bis(amide) and H2. This step occurs without competing protolytic ligand loss – exploiting the limited basicity of the supporting [(boryl)O]− donors. In terms of amide transfer reactivity, the utility of K[{(HCDippN)2BO}2Al(NH2)2] in delivering one or both equivalents of [NH2]− to electrophilic substrates (HBpin, CO2 and Ph2CO) has been demonstrated.
The development of zero-carbon fuel technology is essential for decreasing dependence on fossil fuels and mitigating CO2 emissions.1 A promising avenue within this field is the ′nitrogen economy,′ which focuses on nitrogen-based fuels for large-scale energy storage solutions.1c-1e Among these, ammonia (NH3) is particularly notable due to its high energy density, ease of storage, and versatility as both a hydrogen carrier and fuel.1c-1e Advancing the ammonia economy hinges on improving the synthesis and utilization of NH3.1 The development of efficient catalysts is crucial for synthesizing ammonia from nitrogen (N2) and hydrogen (H2), potentially making the energy-intensive Haber-Bosch process more efficient by emulating the biological nitrogen fixation performed by nitrogenase enzymes.2 Additionally, developing catalysts for the oxidation of ammonia is critical to effectively harnessing the energy stored in N−H bonds.1a However, this process faces a number of challenges, including the stability of Werner-ammine coordination complexes,3 and the high dissociation energy of the N−H bond (416 kJ mol−1).4
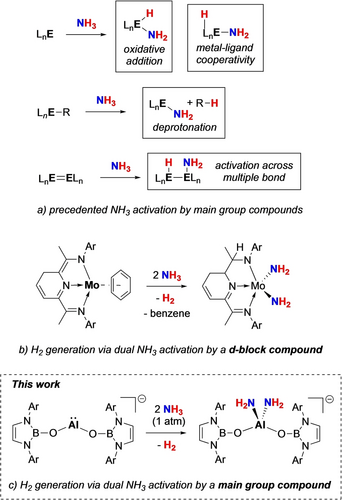
Recent research has focussed productively on transition metal-mediated N−H bond cleavage reactions through various strategies, including coordination-induced bond weakening, hydrogen atom abstraction, inter- and intramolecular deprotonation, oxidative addition, σ-bond metathesis, and electrochemical processes to convert NH3 into N2 and H2.1a, 1b, 4, 5 These advances are vital for developing sustainable energy storage technologies and realizing a functional nitrogen/ammonia economy.
In contrast to d-block systems, however, the exploration of molecular main group complexes for the conversion of NH3 to H2 remains relatively under-explored, despite the fact that NH3 activation by low-valent main group species has been known for nearly 20 years (Scheme 1a).1a, 6 Notable examples include carbenes,6a, 7 low-oxidation-state compounds of the heavier Group 13 and 14 elements,8 multiply bonded species,9 geometrically constrained and low-valent Group 15 compounds,10 and even frustrated Lewis pairs (FLPs).11 More recently, single-electron transfer from a dithiolene zwitterion,12 or from a Bi(II) complex has been reported in the context of NH3 activation,13 although these methods are limited in their ability to subsequently transfer the primary amide (−NH2) moiety. In a recent discovery, Breher et al. demonstrated NH3 splitting using an ambiphilic system and subsequent catalytic transfer to a range of organic substrates, marking a significant advance in the field.14
Activation of NH3 by main group and transition metal compounds [Ar=C6H3-2,6-iPr2].
Aluminium, the most abundant metal in Earth's crust, has shown recent promise in small molecule activation, with both neutral and anionic Al(I) complexes shown to be capable of activating species such as H2, CO, CO2 and N2O.6d, 15 However, NH3 activation by low-valent aluminium compounds remains rare. To date, only one example of NH3 oxidative cleavage - effected by a cyclic(alkyl)(amino)aluminyl system - has been reported.8k Recently, we expanded the range of ligand frameworks available for supporting Al(I) compounds: N-heterocyclic boryloxy (NHBO)-ligated aluminyl compounds could be accessed by ligand metathesis and shown to be competent in the reversible [4+1] cycloaddition of benzene.16 Here, we present NHBO-aluminyl reactivity towards ammonia, including the first example of a molecular main group compound achieving dual ammonia activation and conversion of NH3 to H2. This chemistry also facilitates onward mono and bis(amide) (−NH2) transfer to Ph2CO, CO2 and HBpin. In the case of benzophenone, selective amide transfer leads to a rare crystallographically characterized (metal-masked) carbinolamine,17 a key class of intermediate in imine formation from an amine and an organic carbonyl compound.
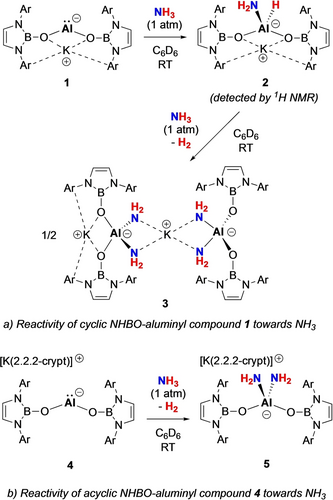
Exposure of a C6D6 solution of K[{(HCDippN)2BO}2Al] (1) to NH3 at 25 °C initially results in the formation of K[{(HCDippN)2BO}2Al(H)(NH2)] (2) through the insertion of Al(I) into the N−H bond of ammonia (Scheme 2). In situ 1H NMR spectroscopy confirms the generation of 2, with a signal at δH=−1.79 ppm (2H) being diagnostic of an Al−NH2 moiety.18 Subsequently, 2 is transformed over 0.5 h by reaction with a second equivalent of NH3, resulting in the formation of the aluminium bis(amide) complex K[{(HCDippN)2BO}2Al(NH2)2] (3) and H2. In this case in situ 1H NMR analysis confirms the generation of H2 (δH=4.47 ppm, Figure S7); similar H2 evolution has previously been reported in the reactions between aluminium hydrides of the type [ArAlH2]2 and ammonia.18a
Activation of NH3 by NHBO-supported aluminyl compounds.
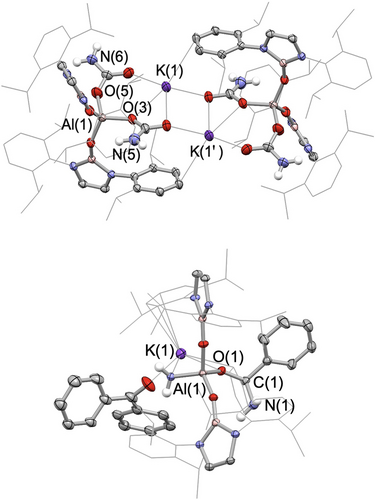
Compound 3 can be isolated in 77 % yield as a white powder and single crystals could be obtained from benzene solution. 3 was fully characterized by multinuclear NMR spectroscopy, elemental microanalysis, and X-ray diffraction. In the 1H NMR spectrum, a 4H signal at −2.15 ppm is assigned to the aluminium-bound NH2 group, i.e. downfield of previously reported aluminium primary amide complexes (δH=−0.68 to −0.22 ppm).18 The molecular structure of compound 3 in the solid state (Figure 1) reveals a dinuclear arrangement featuring two inequivalent tetra-coordinate aluminium centres. Each aluminium centre is bonded to two NHBO ligands and two NH2 fragments. The Al−N bond lengths, ranging from 1.797(4) to 1.821(4) Å, are similar to those measured previously for Al(III) bis(amide) complexes (1.788(2)–1.790(2) Å).18b, 19 Notably, the two K+ cations in 3 exhibit distinct coordination environments: one is encapsulated between two oxygen atoms (thereby constituting a 4-membered AlO2K core), while the other is coordinated by four NH2 units.
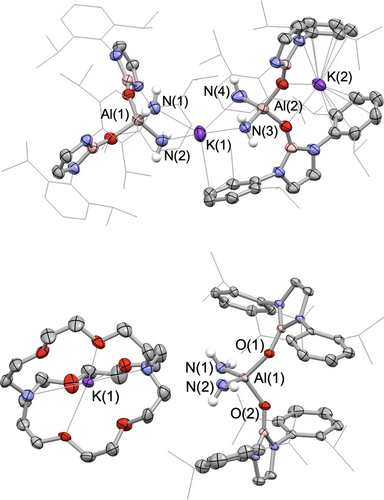
Molecular structures of 3 (upper) and 5 (lower) in the solid state as determined by X-ray crystallography (ellipsoids set at 40 % probability level; H atoms (except for N-bonded Hs) omitted and selected Dipp/iPr groups shown in wireframe format for clarity.
The presence of two −NH2 groups at each metal centre in 3 is consistent with the activation of two equivalents of ammonia at each aluminium. As such, it represents the first example of single-site main group element-mediated dual activation of NH3, and only the second example involving any metal.20 A superficially comparable process involving the conversion of two NH3 molecules to −NH2 ligands with accompanying hydrogen evolution, was reported for the molybdenum bis(imino)pyridine complex, (iPrPDI)Mo(η6-C6H6) (Scheme 1b; iPrPDI=2,6-(DippNCMe)2C5H3N), via a process which exploits metal-ligand cooperativity.20 It is noteworthy that aluminium(III) diamide compounds featuring two terminal NH2 groups, are rare: Roesky and co-workers, for example, reported the synthesis of {HC(MeCDippN)2}Al(NH2)2 via the direct reaction of the corresponding Al(III) dichloride with excess ammonia.18b, 19
The role of the counter cations has been shown to be crucial in aluminyl-mediated small molecule activation, with different reaction pathways and/or regioselectivities, for example, being observed in the presence/absence of a K+ counterion.21 To probe the role of K+ in the current context, we treated the ‘naked’ acyclic aluminyl 4 (in which K+ is encapsulated within 2.2.2-cryptand) with NH3 (Scheme 2b). This chemistry leads to the formation of a similar aluminium bis(amide) complex 4 (plus H2) albeit with a mono-nuclear structure in the solid state (Figure 1), suggesting that the K+ cation does not play a decisive role in this specific activation process.
The reactivity of aluminyl compounds 1 and 4 towards ammonia differs from that determined for Kinjo's cyclic C,N-chelated aluminyl system, [{(Me3Si)2C(CH)2NAd}Al]−.8k 1/4 activate two ammonia molecules and liberate H2, while the latter is reported to undergo a single ammonia activation step involving the insertion of Al(I) into the N−H bond. In a broader context, we hypothesized that the nature of the Al−H bond formed in the first activation step might influence the feasibility of the second (hydrogen evolution) process. With this in mind, and given the isoelectronic relationship between anionic aluminyl and charge-neutral silylene systems, we examined the reactivity of bis(boryloxy)silylene 6 towards NH3, hypothesizing that a less hydridic Si−H bond would be less prone to formal protonolysis by a second equivalent of NH3.22 Consistently, under comparable conditions to those used for 1/4, this chemistry generates the ‘simple’ oxidative addition product 7 via insertion of the Si(II) centre into the N−H bond (Scheme 3 and Figure 2). Further treatment of compound 7 with excess ammonia under forcing conditions did not result in dual ammonia activation.
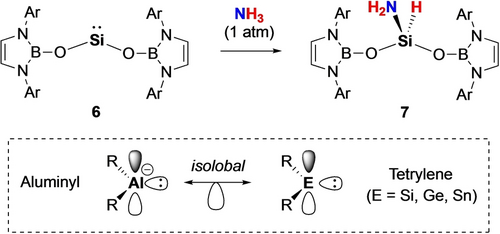
Reactivity of silylene 6 towards ammonia; isoelectronic relationship between anionic aluminyl and charge-neutral tetrylene species.
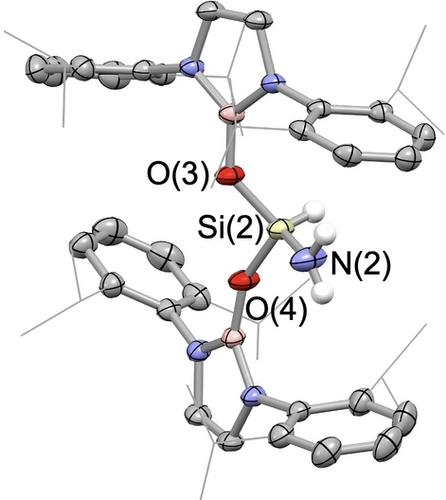
Molecular structure of one of the molecules in the asymmetric unit of compound 7 in the solid state, as determined by X-ray crystallography (thermal ellipsoids set at 40 % probability). For clarity, NHBO-bound hydrogen atoms have been omitted, and iPr groups are represented in a wireframe format.
The mechanistic steps implicit in the conversion of the anionic component of aluminyl 4 to bis(amide) 5 (and related systems) have been investigated by quantum chemical calculations (PCM-PBE0-GD3BJ/Def2-TZVP//PBE0-GD3BJ/Def2-SVP). These calculations show that a two-step process involving initial N−H activation at Al(I), followed by protonolysis of the resulting Al−H bond is feasible, with associated activation barriers of 115.4 and 120.4 kJ mol−1, respectively (Figure 3). The overall thermodynamics associated with the two steps are strongly exergonic (−148.8 and −65.8 kJ mol−1, respectively). Moreover, the relative energetic barriers associated with the formation and consumption of (amido)hydride complex 2− are consistent with the observation (by NMR spectroscopy) of 2 in solution at short reaction times.
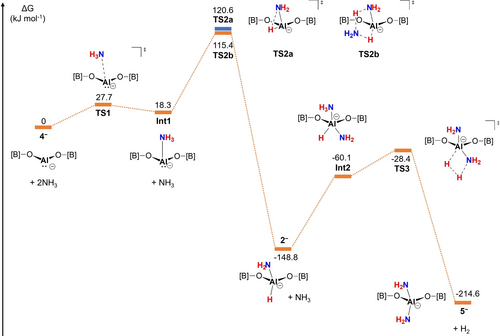
DFT-calculated reaction profile for the conversion of the anionic component of 4 (plus two molecules of ammonia) to 5 (PCM-PBE0-GD3BJ/Def2-TZVP//PBE0-GD3BJ/Def2-SVP). [B]=-B(NDippCH)2.
For the N−H activation step, we considered two possible mechanistic variants through which an initially-formed NH3 adduct (Int1) could be converted into the corresponding (amido)hydride via proton transfer from N to Al. Accordingly, we find that a bimolecular reaction involving a second molecule of ammonia acting as a proton shuttle incurs a lower energetic cost (via TS2b), than the corresponding reaction involving a single NH3 molecule and a three-centre transition state (i.e. TS2a). In this respect, the initial N−H activation step mirrors those reported for NH3 activation by isoelectronic group 14 systems.8b, 8g, 8j, 8n, 23
The observation that aluminyl complexes 1/4 activate two molecules of NH3 – while silylene system 6 effects only the initial N−H oxidative addition process – speaks to the feasibility of the second protonolysis step. Consistently, the barrier associated with accessing the AlHHN-containing TS3 (120.4 kJ mol−1), is significantly lower than that calculated for the corresponding silicon-containing system (217 kJ mol−1; Figure S23). This difference can be related in turn to the less hydridic nature of the silicon-bonded hydride, which bears a significantly less negative partial charge than its aluminium-bonded counterpart (Mulliken charges: −0.05 vs. −0.23 e). Interestingly our calculations also suggest that Kinjo's amido/hydride [K(12-crown-4)2] [{(Me3Si)2C(CH)2NAd}Al(H)(NH2)] should react in a thermodynamically favourable (ΔGo=−25 kJ mol−1) and kinetically facile manner (ΔG≠=114 kJ mol−1) to generate the corresponding bis(amide) and dihydrogen. Noting the low temperature conditions utilized in this study (and in the absence of any experimental data on the further reaction with NH3),8k it is conceivable that the onward reaction instead leads to protolytic loss of the C,N-donor supporting ligand scaffold. In the case of 2 the less basic nature of the O-donor supporting scaffold would be expected to render protolytic ligand loss less favourable, and we see no evidence for the formation of (HCDippN)2BOH.
Finally, we set out to explore whether doubly activated bis(amide) 3 could be used to deliver the −NH2 fragment to unsaturated and/or electrophilic substrates. As such, we probed the chemistry of 3 towards the carbonyl-containing systems CO2 and Ph2CO, and the borane HBpin. The reaction with CO2 leads to transfer of both amide moieties (to two CO2 molecules), yielding bis(carbamate) complex 8 (Scheme 4). Compound 8 has been characterized by multinuclear NMR spectroscopy and X-ray diffraction (Figure 4). On the other hand, the reaction of compound 3 with more sterically encumbered substrate Ph2CO leads to transfer of a single amide unit, despite the presence of an additional molecule of ketone in the product (Figure 4). The resulting product (9) contains a (unique) metalated carbinolamine fragment – the protonated form of which is critical intermediate in imine formation from ammonia and a carbonyl compound.17
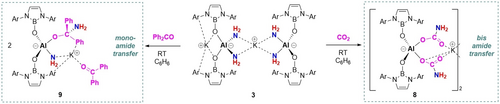
Reactivity studies of compound 3: transfer of one or both amido groups.
Molecular structures of 8 (upper) and 9 (lower) in the solid state as determined by X-ray crystallography (ellipsoids set at 40 % probability level; H atoms omitted and iPr/Dipp groups shown in wireframe format for clarity).
In addition, we investigated the reactivity of compound 3 towards (hydridic) B−H bonds with the aim of regenerating the Al−H bond present in intermediate 2. The reaction with pinacolborane (HBpin) does indeed generate pinBNH2 (presumably driven thermodynamically by B−N bond formation), as confirmed by 11B{1H} NMR monitoring.24 However, 1H NMR data implies a mixture of aluminium-containing products (which could not easily be separated), potentially derived from 3 by the substitution of variable numbers of −NH2 groups by H.
In conclusion, we report a novel advance in main-group-mediated ammonia activation and conversion, demonstrating the efficacy of aluminyl compounds in both the activation of NH3 and the generation of H2 (along with an aluminium(III) bis(amide)) on uptake of a second molecule of ammonia. Critically, this chemistry is facilitated by the accessibility of a (coordination/proton shuttling) N−H oxidative addition pathway, and by the inertness of the NHBO-supporting ligand set in the subsequent protonolysis step. As such, we demonstrate the capabilities of low-valent main group complexes in a topical transformation previously precedented by only a single transition metal system.
Acknowledgments
DS is thankful to the Deutsche Forschungsgemeinschaft (DFG) for financial support (Walter Benjamin Fellowship). LG, and JS thank the EPSRC for studentship funding through the OxICFM CDT (EP/S023828/1). PV wishes to acknowledge the Research Council of Finland (grants 338271 and 359912) for funding and CSC – IT Center for Science, Finland, for computational resources.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.