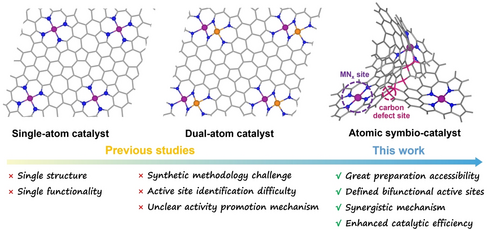
Atomic Symbiotic-Catalyst for Low-Temperature Zinc-Air Battery
Graphical Abstract
A new “atomic symbiotic-catalyst” system comprising single-atom MNx and local sp3 carbon defects is proposed, which marries the advantages of typical single-/dual-atom catalysts while addressing their respective weaknesses, thus synergistically achieves high electrocatalytic activity and battery efficiency.
Abstract
Atomic-level designed electrocatalysts, including single-/dual-atom catalysts, have attracted extensive interests due to their maximized atom utilization efficiency and increased activity. Herein, a new electrocatalyst system termed as “atomic symbiotic-catalyst”, that marries the advantages of typical single-/dual-atom catalysts while addressing their respective weaknesses, was proposed. In atomic symbiotic-catalyst, single-atom MNx and local carbon defects formed under a specific thermodynamic condition, act synergistically to achieve high electrocatalytic activity and battery efficiency. This symbiotic-catalyst shows greater structural precision and preparation accessibility than those of dual-atom catalysts owing to its reduced complexity in chemical space. Meanwhile, it outperforms the intrinsic activities of conventional single-atom catalysts due to multi-active-sites synergistic effect. As a proof-of-concept study, an atomic symbiotic-catalyst comprising single-atom MnN4 moieties and abundant sp3-hybridized carbon defects was constructed for low-temperature zinc-air battery, which exhibited a high peak power density of 76 mW cm−2 with long-term stability at −40 °C, representing a top-level performance of such batteries.
Introduction
Renewable energy technologies, such as fuel cells and metal-air batteries, are being vigorously pursued in the current era of heightened focus on sustainability.1 As the cornerstone of these technologies, electrocatalysts must meet stringent requirements for low cost and high performance, especially when considering large-scale applications. Atomic-level electrocatalyst design has, therefore, attracted extensive interests due to its merits of much more sustainable ways in maximizing atom utilization efficiency and increasing the activity per metal atom. “Single-atom catalysis” and the subsequently emerging “dual-atom catalysis”, both strictly adhering to the principles of electrocatalyst design atom-by-atom, have become the most popularized concepts in electrocatalysis.2 In relevant studies, metal-nitrogen-carbon (M−N−C) materials are the most representative electrocatalyst system, predominantly employed for oxygen reduction reaction (ORR) in fuel cells and metal-air batteries.3 Due to their structural similarity to the oxygen-binding site in cytochrome c oxidase, single-atom M1Nx, especially those centered around iron, offer substantial ORR activity.4 Recent studies have further demonstrated that paired metal atoms, M1M2Nx, exhibit strong electronic coupling that optimizes electrochemical performances. And more importantly, they readily drive complete ORR through cooperative binding and consecutive reactions.5 Many pairs of metal atoms, such as cobalt-platinum,6 zinc-cobalt,7 iron-iron,8 and iron-selenium,9 have been found to be highly active for ORR.
These M1M2Nx electrocatalysts, although promising, encounter a synthetic challenge associated with maximizing the proportion of paired metal atoms with well-defined dinuclear active sites in the prepared product. Since more than 20 possibilities exist for the geometric structure of a given M1M2Nx,10 it is difficult to identify which one is truly catalytically active and which are inert or inactive for the ORR. In addition, an even greater issue confronts the community in revealing the key mechanism responsible for the superior performances of paired atoms compared to conventional single atoms. Some researchers attributed the activity enhancement to the nearest-neighbor interaction between paired metal atoms, while others emphasized the role of sub-nanometer inter-site distances, in which two metal centers catalysed different primary steps separately.4a, 11 Such current limitations make it challenging to design an efficient M1M2Nx catalyst with clear structure–activity relationships at the atomic level. An innovative electrocatalyst system that marries the advantages of typical single-atom and dual-atom catalysts while addressing their respective weaknesses shall be much more realistic and achievable.
Herein, we proposed a catalytic concept termed as “atomic symbiotic-catalyst (ASC)”, where single-atom MNx sites and local carbon defects (Cd), formed under a specific thermodynamic condition, act synergistically to achieve high electrocatalytic activity and high battery efficiency. Considering the number of metal atoms involved, ASC resembles “single-atom catalyst” with only one metal site. Functionally, ASC shares more similarities to “dual-atom catalyst” in its utilization of two active sites having a mutually beneficial interaction. Importantly, as shown in Figure 1, ASC not only shows great structural precision and preparation accessibility owing to their reduced complexity in chemical space, but also outperforms the intrinsic activities of conventional single-atom catalysts with a multi-active-sites synergistic effect. In addition, compared to conventional defect-engineered SACs, the one-step synthesis of ASC would enhance the defect-metal electronic interactions and elevate the intrinsic activity in multi-electron/proton transfer reactions due to the synergistic activation pathways. In our proof-of-concept study, an ASC comprising MnN4 moieties and abundant sp3-hybridized carbon defects (i.e., MnN4/Cd ASC) was constructed. With symbiotic active sites, we obtained an exceptionally active electrocatalyst for ORR, leading to a flexible zinc-air battery (ZAB) with a top-level peak power density at room temperature and low temperature (up to −40 °C).
The structural feature comparison of single-atom catalyst, dual-atom catalyst and atomic symbiotic-catalyst.
Results and Discussion
Catalyst Synthesis and Characterization
As one kind of low-cost biomass waste, the membrane of gizzard (MG) from killed chicken consists of keratin derived from polycondensation of multi-amino acids (e.g., alanine, proline, glycine, methionine, etc.) with abundant C, N, and O atoms, which can be utilized as a good precursor for synthesizing heteroatom-doped carbon-based ASCs. Recently, Mn was widely chosen for constructing ORR active sites due to its combination of multifarious properties and advantages, including unique electronic structure, inhibition of Fenton reaction, cost-effectiveness, environmental compatibility, good stability, etc., which make it a promising alternative to other transition metals in fuel cell and metal-air battery applications.3e, 4a, 12 Therefore, in this work, we choose MG as the biomass precursor and Mn as the active single-atom sites to construct the ASCs. As shown in Figure 2a, N groups provided abundant adsorption sites to anchor Mn2+ ions during the impregnation process in MnCl2 solution. During the carbonization treatment at 700–900 °C, thermal decomposition of organic compounds and formation of fundamental carbon skeleton simultaneously took place. Mn2+ was decomposed to Mn-based nanoparticles and single atoms anchored by N coordination. As demonstrated by electron microscope analysis (i.e., scanning electron microscope (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM)) and energy-dispersive X-ray spectroscopy (EDX) mapping tests shown in Figures S1 and 2, after carbonization treatment at 800 °C, the sample shows a plate-like structure, and Mn-based nanoparticles and clusters can be observed on the N-doped carbon host (MnNPs-NC). Meanwhile, MnCl2 pyrolysis created homogeneous erosion on graphitic layer to generate abundant micropores within the plate-like structures, which can provide large specific surface area and fast ion transportation for electrochemical reactions.13 Then, Mn-based nanoparticles were removed by acid pickling and only single-atom sites were preserved. Interestingly, the coordination structure of Mn single atoms varied with the pyrolysis temperature. MnN5, MnN4 and MnN3 moieties were obtained at 700, 800 and 900 °C, respectively. Moreover, the amount of carbon defect sites was also greatly affected by the pyrolysis temperature, among which the sample carbonized under 800 °C possesses the most carbon defective sites (i.e., MnN4/Cd ASC) compared to those of the 700 and 900 °C samples (i.e., MnN5/Cd and MnN3/Cd ASC, respectively). Figures 2b, 2c, and Figures S3a, 3b, 4a, 4b show the SEM and TEM images of MnN4/Cd, MnN5/Cd and MnN3/Cd samples. All the samples possess disordered plate-like structure with diameter ranging from hundreds of nanometers to several micrometers. The HRTEM images and corresponding selected area electron diffraction (SAED) patterns (Figures 2d, S3c and S4c) of the samples show the disordered texture structures with some obscure ribbons, indicating a certain degree of graphitization,14 as also testified by the powder X-ray diffraction (XRD) results. The XRD patterns of all MnNx/Cd (x=3, 4, 5) samples shown in Figure S5 exhibit a broad peak at 25.2° and a hump-like peak at 42.6°, which can be assigned to the (002) and (100) diffraction planes of graphitic frameworks (JCPDS no. 41–1487), respectively. No bright nanoparticles or clusters are present in the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and elemental mapping images (Figures 2e, 2 f, Figures S6 and S7), which can also be ascertained by their corresponding SAED patterns, indicating that Mn, N and C species are uniformly distributed within the carbon skeletons. For comparison, the nitrogen-doped carbon (NC) sample prepared without MnCl2 addition was also characterised, which showed similar morphology and graphitization structures with the MnNx/Cd samples (Figures S8 and S9). In addition, the aberration-corrected HAADF-STEM (AC-HAADF-STEM) images of MnN4/Cd (Figure 2g), MnN5/Cd (Figure S10) and MnN3/Cd (Figure S11) samples show a number of individual bright spots as marked with green circles, which elucidate the presence of isolated Mn atoms anchored on the N-doped carbon nanosheets.15 The mass loadings of Mn in MnNPs-NC, MnN5/Cd, MnN4/Cd and MnN3/Cd was determined to be 5.9 wt %, 1.7 wt %, 1.3 wt %, 1.0 wt %, respectively, from the results of inductively coupled plasma mass spectrometer (ICP-MS). The surface area and porosity properties of the obtained samples were investigated by N2-adsorption/desorption measurements (Figure S12). All of the samples show similar microporous structures with pore distribution below 3 nm, as well as the close Brunauer–Emmett–Teller (BET) specific surface areas of 662.0, 621.9 and 491.3 m2 g−1 for MnN5/Cd, MnN4/Cd and MnN3/Cd, respectively, which are much higher than those of NC (19.1 m2 g−1) and MnNPs-NC (54.9 m2 g−1) due to the MnCl2 activation and subsequent acid pickling treatments (Figure S13).
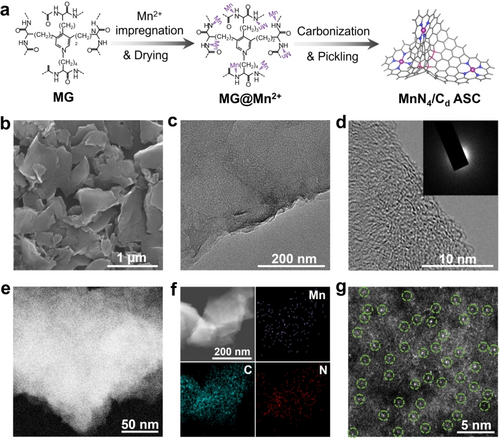
Synthesis and structural characterization of the MnN4/Cd ASC. (a) Schematic illustration of the MG precursor strategy to synthesize the MnN4/Cd ASC. (b) SEM, (c) TEM, (d) HRTEM and SAED pattern of MnN4/Cd ASC. (e) HAADF-STEM image of MnN4/Cd ASC. (f) Element maps of MnN4/Cd ASC, Mn (purple), C (blue), N (red), respectively. (g) AC-HAADF-STEM image of MnN4/Cd ASC.
To identify the local electronic and geometric structures of Mn species, X-ray absorption fine structure (XAFS) measurements were systematically performed as shown in Figure 3. The X-ray absorption near-edge spectroscopy (XANES) (Figure 3a) features of the MnNx/Cd samples are obviously different from Mn foil and Mn2O3, indicating their varied atomic structures. Nevertheless, the nearly coincident near-edge absorption. energies (E0) of MnNx/Cd samples and MnO reveals that the oxidation states of Mn atoms are close to Mn2+. Figure 3b presents the R-space data of k3-weighted Mn K-edge Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) spectra of MnNx/Cd and the reference samples. All three MnNx/Cd samples exhibit only one obvious FT peak located at about 1.6 Å attributed to the Mn−N coordination scattering. No peaks of Mn−Mn or Mn−O−Mn coordinations are observed compared to Mn foil, MnO and Mn2O3, demonstrating the isolated dispersion of Mn atoms on the NC substrate. As the Mn K-edge wavelet transform (WT)-EXAFS of the samples illustrated in Figure 3c and Figure S14, the WT contour plots of MnNx/Cd samples display only one intensity maximum of Mn−N coordination at ~5 Å−1, without other scattering paths comparing with the Mn foil WT plots. The above results further demonstrate the formation of Mn single-atom sites. To extract the precise structural parameters, quantitative FT-EXAFS fitting was performed and the results were exhibited in Figure 3d-f and Table S1. The first shell of the central Mn atom displayed Mn−N coordination numbers of 5.4, 4.3 and 2.8 with mean bond lengths of 2.17, 2.18 and 2.15 Å for MnN5/Cd, MnN4/Cd and MnN3/Cd, respectively. In addition, as shown in the N 1s X-ray photoelectron spectroscopy (XPS) results in Figure 3g, the spectra consist of three fitting peaks at 398.7, 400.3 and 401.9 eV arising from the pyridinic-N, pyrrolic-N, and graphitic-N, respectively, and pyrrolic-N is the primary N species for all the three samples.16 Therefore, we can infer that the Mn atoms mostly coordinated with pyrrolic-N atoms to form single-atom MnN5, MnN4 and MnN3 moieties on the NC matrix for MnN5/Cd, MnN4/Cd and MnN3/Cd, respectively. For MnN5 site, the Mn single atom could be coordinated by four surface pyrrolic-N atoms and one axial N ligand.
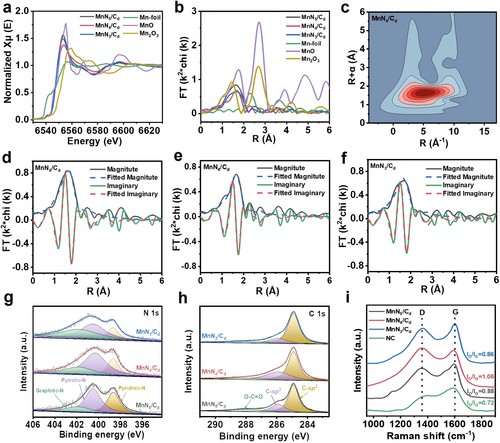
Microenvironment characterization of the MnNx/Cd ASCs. (a) Mn K-edge XANES spectra. (b) Mn K-edge FT-EXAFS spectra. (c) WT-EXAFS of MnN4/Cd ASC. (d) EXAFS R space fitting curves of MnN5/Cd ASC. (e) EXAFS R space fitting curves of MnN4/Cd ASC. (f) EXAFS R space fitting curves of MnN3/Cd ASC. (g) N 1s and (h) C 1s XPS spectra of the MnNx/Cd ASCs. (i) Raman spectra of the NC and MnNx/Cd ASCs.
The carbon defects and graphitization characteristics of the MnNx/Cd ASCs were further investigated by the C 1s XPS and Raman spectra analysis. As shown in Figure 3h, the C 1s spectra can be deconvoluted into three peaks centered around the binding energies of 284.9, 285.9 and 288.0 eV, corresponding to sp2-hybridized C, sp3-hybridized C and O-C=O bonds, respectively.17 Notably, MnN4/Cd sample shows the highest content of sp3-hybridized C species (33.3 %) compared to MnN5/Cd (23.8 %) and MnN3/Cd (18.5 %), indicating the most sp3-hybridized defect sites in MnN4/Cd. As shown in the Raman spectra of Figure 3i, all the samples display two intensive peaks centered at the wavenumber of 1360 cm−1 (D-band) and 1600 cm−1 (G-band). The D-band signifies the disordered structures induced by the carbon lattice defects, while the G-band refers to the stretching vibration of sp2-hybridized C−C bond in graphitic structure.18 The ID/IG values of NC, MnN5/Cd, MnN4/Cd and MnN3/Cd are 0.72, 0.88, 1.06 and 0.86, respectively, indicating an enhanced degree of disorder for MnN4/Cd with the most defective sites. Based on the above discussion, we know that the varying pyrolysis temperature can not only tune the coordination structure of Mn single-atom sites with MnN5, MnN4 or MnN3 moieties, but also regulate the carbon defect amount and structure, which endows the products great potentials as superior ASCs for energy conversion and storage applications.
Electrochemical and ZAB Performance
The ORR properties of MnNx/Cd ASCs were investigated with a typical three-electrode system in O2-saturated 0.1 M KOH electrolyte. The polarization curves and Tafel plots are shown in Figure 4a, and Figure S15–S17, and the corresponding mass activities and half-wave potentials (E1/2) are summarized in Figure 4b. These results demonstrate that the optimal ORR performance is achieved by MnN4/Cd ASC with MnN4 coordination and most carbon defective sites, which delivers the minimal Tafel slope of 42 mV dec−1, the highest mass activity of 120.4 mA mgmetal−1 and the most positive E1/2 of 0.900 V compared with the MnN5/Cd, MnN3/Cd, MnNPs-NC, NC and Pt/C counterparts. Moreover, the ORR performance is better than many recently reported non-noble metal based single-atom catalysts (Table S2). The ORR pathway of MnN4/Cd was evaluated by the rotating disk electrode (RDE) measurements. As shown in Figure S18a, the corresponding Koutecky–Levich (K–L) plot is linear, and the value of electron-transfer number (n) is calculated to be about 4.0 over the potential range from 0.3 to 0.8 V, suggesting the first-order reaction kinetics toward the concentration of dissolved oxygen and the four-electron ORR pathway. The methanol tolerances of MnN4/Cd and commercial Pt/C were also tested by injecting methanol solution to the electrolyte during the chronoamperometric measurements. As shown in Figure S18b, after methanol injection, MnN4/Cd still maintains a stable current density, while the Pt/C exhibits a sharp jump due to the methanol oxidation, revealing the excellent tolerance to methanol crossover of MnN4/Cd sample. Moreover, in the accelerated durability test (Figure 4c), no obvious half-wave potential decay can be observed even after 15000 continuous cycles, demonstrating the superior long-term durability of MnN4/Cd ASC. To reveal the N coordination number effect on the intrinsic activity of Mn single-atom sites, we studied the ORR mechanism on MnNx (x=3, 4, 5) sites by employing density-functional-theory (DFT) calculations. According to the results in Figure S19, MnN3 shows a higher energy barrier for the hydrogenation of OO (1.59 eV) than MnN4 (1.21 eV), indicating the poor activity of MnN3. Besides, MnN5 shows a positive free energy of *OO (0.26 eV), indicating that the adsorption of OO is endothermic on MnN5. Thus, the MnN4 shows the best catalytic activity in ORR among all MnNx systems, which agrees well with the experimental results.
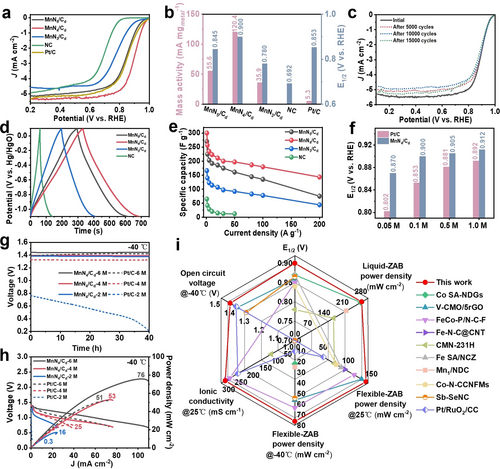
Electrochemical and ZAB performances of MnNx/Cd ASCs. (a) ORR polarization curves of MnNx/Cd and reference catalysts in 0.1 M KOH. (b) Comparison of mass activities and E1/2 of MnNx/Cd and reference catalysts. (c) ORR polarization curves of initial MnN4/Cd and after 5000, 10000 and 15000 potential cycles. (d) The comparison of GCD curves at 1 A g−1 for the MnNx/Cd and NC samples measured in three-electrode configuration using 6 M KOH as electrolyte. (e) Plots of specific capacitance versus current density for the MnNx/Cd and NC samples. (f) Comparison of E1/2 for MnN4/Cd and 20 % Pt/C in KOH electrolytes with different concentrations. (g) Open circuit voltage plots of flexible solid-state ZABs based on MnN4/Cd and Pt/C as air cathodes with different concentrations (6, 4, 2 M) of KOH electrolyte at −40 °C. (h) Power density curves of the flexible solid-state ZABs at −40 °C. (i) Comprehensive comparison of the ZAB performances based on MnN4/Cd and other reported state-of-the-art catalysts. References are provided in Table S3 (highlighted in blue).
To verify the ion enrichment effect of carbon defects through the electric double layer capacitance mechanism, we further tested the supercapacitive performance of the MnNx/Cd and NC samples by cycling voltammetry (CV) and galvanostatic charge–discharge (GCD) in the three-electrode and two-electrode systems (Figure 4d and Figures S20–S27). The GCD curves of MG-derived samples at the current density of 1 A g−1 are shown in Figure 4d. In comparison with other samples, MnN4/Cd displays larger specific capacitance as ascertained by its longer discharge time. In addition, MnN4/Cd exhibits linear symmetrical charge/discharge profiles with negligible voltage drops in the potential range of −1.1–0.1 V, which indicates a small internal resistance and good reversible capacitive behavior. As shown in Figure 4e, the specific capacitances of the samples at different current densities were calculated from their corresponding GCD curves. Notably, MnN4/Cd presents the maximal specific capacitance (300.2 F g−1 at 1 A g−1) as compared to other samples. Even at an ultrahigh current density of 200 A g−1, the MnN4/Cd still retains a specific capacitance of 143.5 F g−1, which is almost as twice-fold as that of MnN5/Cd (e.g., 75.1 F g−1) and more than triple-fold as those of MnN3/Cd (e.g., 44.3 F g−1). In contrast, NC displays a low specific capacitance of 65.1 F g−1 at 1 A g−1 and only 19.2 % of capacitance retention at 50 A g−1 (compared with 1 A g−1). All the supercapacitive results thoroughly demonstrate the superior electric double layer capacitance of MnN4/Cd with the most sp3-hybridized carbon defect sites compared with those of MnN5/Cd and MnN3/Cd with less defective carbon sites. For further comparison, the carbonization temperature was increased to 1000 °C to prepare the Mn-NC-1000 sample, which shows the similar morphology, graphitization degree and diffraction peaks as those of MnNx/Cd ASCs, and the Mn atoms are uniformly dispersed on the NC support (Figure. S28 and S29). However, the amount of sp3-hybridized C (16.1 %) species decreases significantly due to the excessive carbonization temperature (Figure S30a). Moreover, the pyrrolic-N species greatly decreases and the Graphitic-N become the primary N species (Figure S30b). As a result, Mn-NC-1000 delivers a E1/2 of 0.8 V for ORR (Figure S31) and a low specific capacitance of 126.4 F g−1 at 1 A g−1 for supercapacitor (Figure S32). The poor electrochemical performances of Mn-NC-1000 as compared to MnNx/Cd ASCs could be caused by its modified N coordination structure and less sp3-hybridized carbon defect sites. To demonstrate the ion enrichment effect of MnN4/Cd sample on the ORR activity, we compared its ORR performance with Pt/C in KOH electrolytes with different concentrations from 0.05 to 1.0 mol L−1. As shown in Figures 4f and S33, along with the KOH concentration decreases from 1.0 mol L−1 to 0.05 mol L−1, the E1/2 value of Pt/C decreases from 0.892 V to 0.802 V with 90 mV fall-off due to the lowered ionic conductivity of electrolyte. Meanwhile, the MnN4/Cd sample shows only 42 mV decrement of E1/2 value from 0.912 V to 0.870 V. This phenomenon reveals the positive influence of the ion enrichment effect from carbon defects of MnN4/Cd on the ORR performance. Moreover, the LSV curves show a decreased limiting current density along with the increasing KOH concentration, which can be due to the reduced dissolved oxygen concentration in high ion-concentration electrolyte.
To further verify the influence of ion enrichment on ZAB performances, concentration effect (i.e., 6 M, 4 M and 2 M) of KOH electrolyte on the performance of MnN4/Cd-based ZABs was scrutinized. It is known that the 6 M KOH electrolyte of ZABs delivered maximum electrolyte conductivity, while electrolytes possessing reduced concentration (e.g., 4 M and 2 M) exhibited decreased ionic conductivity.19 As illustrated in Figure S34a, the initial open circuit voltages of the MnN4/Cd-based liquid ZABs surpass 1.4 V across varying concentrations of KOH electrolyte. The open circuit voltage of ZAB with a 6 M KOH electrolyte remains virtually unchanged even after 120 hours. Remarkably, the ZAB open circuit voltage decreases only 0.06 and 0.12 V, respectively, in the cases of 4 and 2 M KOH concentrations. In sharp contrast, the open circuit voltages of the liquid ZABs utilizing the commercial Pt/C standard sample experience a significant decline as the electrolyte concentration decreases. The power density achieved by the MnN4/Cd-based liquid ZAB with a 6 M KOH electrolyte is measured at 255 mW cm−2 (Figure S34b). Additionally, strong power densities are observed in liquid ZABs with reduced electrolyte concentrations (200 mW cm−2 for 4 M KOH and 128 mW cm−2 for 2 M KOH). On the other hand, the power density of the liquid ZAB composed of the commercial Pt/C standard sample is 140 mW cm−2 at 6 M KOH electrolyte, with a sharp decrease after reducing the electrolyte concentration (54 mW cm−2 at 4 M KOH and 37 mW cm−2 at 2 M KOH). The instantaneous discharge performances in the latter case are significantly lower than that exhibited by MnN4/Cd-based liquid ZABs. These results collectively demonstrate the superior ZABs performance offered by MnN4/Cd catalyst even when used in low-alkalinity electrolyte.
More importantly, the corresponding flexible solid-state ZABs were assembled to further assess the practical potential. A polyacrylic acid (PAA)-based hydrogel solid electrolyte that is resistant to low temperature was developed and soaked with varying concentrations of KOH. By using carbon cloth coated with MnN4/Cd catalyst as the cathode and zinc foil as the anode, we were able to create flexible solid-state ZABs. As shown in Figure S35a, even after 40 hours, the open circuit voltage of MnN4/Cd-based flexible ZABs utilizing solid electrolytes with different KOH concentrations remain stable above 1.35 V. For flexible ZABs composed of Pt/C standard sample, there is a significant decrease in open circuit voltage when electrolyte concentration decreases (i.e., 1.41, 1.3 and 0.98 V for 6, 4 and 2 M, respectively). The MnN4/Cd-based flexible ZAB utilizing a concentration of 6 M has an excellent discharge power density of up to 155 mW cm−2, which is higher than standard samples (115 mW cm−2) (Figure S35b). The electrochemical impedances of MnN4/Cd and Pt/C-based flexible ZABs were measured to be 0.94 and 1.15 Ω (Figure S36), and the corresponding ionic conductivities were calculated to be 283.7 and 231.9 mS cm−1, respectively. Even after reducing electrolyte concentration levels, this type of flexible battery still maintained high power densities (i.e., 88 and 48 mW cm−2 for 4 and 2 M, respectively). In contrast, power densities for flexible ZABs with standard sample significantly declined once their respective concentrations were lowered (e.g., 41 mW cm−2 for 4 M) or could no longer discharge altogether (at just 2 M).
Low temperature tolerance is an important characteristic of ZABs. To this end, at temperature as low as −40 °C, the MnN4/Cd-based flexible ZABs still demonstrated good discharge capacity while maintaining open circuit voltages above 1.35 V and retaining some degree of power density (76 mW cm−2 for the 6 M electrolyte) (Figures 4g and h), which is better than most flexible ZABs currently available, as shown in Table S3. To sum up, MnN4/Cd ASC delivered comprehensive advantages for ZABs (Figure 4i),20 regarding the superior electrocatalytic activity (i.e., mass activities and E1/2 of ORR) and stability, and performance of liquid and flexible ZABs toward wide range of working conditions (e.g., low temperature and low alkalinity)
Mechanistic Study for the Electrochemical Performance by DFT
By employing DFT calculations, we demonstrated the ORR mechanism on MnN4 with the assistance of K+ ion. Based on the electrical double layer effect observed in carbon-based electrodes, cations accumulate near the interface at negative voltages, and we find that sp3-hybridized carbon facilitates this process. Figure 5a shows the atomic configuration of MnN4 on sp2- or sp3-hybridized C, while Figure 5b presents the adsorption energy of K+ ions on the graphene surface at sp2-C and sp3-C sites, as shown in Figure 5a. For sp2-C, the adsorption energies at different sites are all positive, indicating very weak adsorption of K+ ions at open-circuit potential. In contrast, the adsorption of K+ at sp3-C sites is more energetically favorable compared to sp2-C, which can be attributed to the quantum capacitance effect. Furthermore, as the distance between K+ and Mn decreases, the adsorption energy of K+ on sp3-C becomes more negative, suggesting that the MnN4 active site also attracts K+ ions, as shown in Figure 5c.
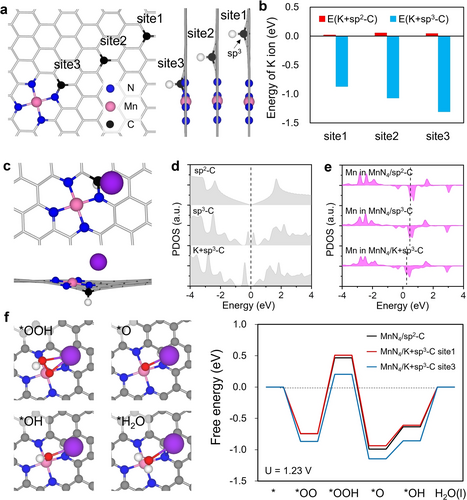
DFT calculation results showing the ORR mechanism with K+ cation. (a) Atomic configurations of MnN4/Cd ASC with sp3 carbon at different sites. Mn and N atoms are marked by pink and blue, respectively. The sp3 carbon is marked by black. (b) Adsorption energies of K+ cation on different sites of MnN4/Cd ASC according to (a). (c) Atomic configuration with the lowest adsorption energy according to (b). K+ cations are marked by purple. (d) PDOS on different carbon atoms. (e) PDOS on Mn ion in different systems, (f) Free energy diagrams of ORR on MnN4/sp2-C (black), MnN4/sp3-C when K+ is at site1 (red), and MnN4/sp3-C when K+ is at site3 (blue). Left panel is the atomic configurations of intermediates during the pathway MnN4/K+sp3-C site3 according to the right panel.
To determine whether sp3-C or the accumulated K+ ions promote ORR catalytic activity, we show the projected density of states (PDOS) for sp2-C and sp3-C with and without K+ cations in Figure 5d, and Figure 5e displays the PDOS for the Mn ion. For sp2-C, a V-shaped dip at the Fermi level is observed, while two peaks appear at the Fermi level for sp3-C, which are the local defect states. Figure S37 shows that sp3-C exhibits a very high CQ, whereas sp2-C contributes little to CQ near zero, consistent with the experimental results (Figure 4d and e). After the adsorption of the K+ ion, the sp3-C becomes n-doped, which is further confirmed by the PDOS on Mn in Figure 5e. Compared with MnN4 located on sp2-C, the PDOS on Mn in MnN4/sp3-C remains unchanged, indicating that the sp3-hybridized C does not significantly affect the electronic properties of Mn. When the K+ ion is located at the sp3-C site, as shown in Figure 5e, the peaks above the Fermi level shift to lower energies, suggesting that the MnN4/K+-sp3-C system exhibits higher catalytic activity. Therefore, the sp3-C accumulates K+ ions, and the presence of these K+ ions near the active site that plays an important role in promoting ORR, in agreement with the experimental findings.
Based on the adsorption energy in Figure 5b, K+ ions prefer to locate near MnN4, suggesting that K+ ions can form bonds with intermediates during the ORR. Figure 5f shows the free energy landscape for the ORR process on MnN4/sp2-C, MnN4/sp3-C with the K+ ion at site1 and site3, respectively. The rate-limiting step in the ORR is the hydrogenation of the oxygen molecule on MnN4, with a barrier of 1.21 V. When the K+ ion is adsorbed on the sp3-C at site1, which is away from the MnN4 active site, the overpotential increases to 1.25 V, similar to that in MnN4/sp2-C. However, when the K+ ion is adsorbed at sp3-C on site3, which is closer to the MnN4 active site, the overpotential decreases significantly to 1.07 V. This decrease is attributed to the weakening of the O−O bond by the adsorbed K+ ion. The O−O bond lengths of the *OOH intermediate on pristine MnN4, MnN4 with K adsorbed at site1, and MnN4 with K adsorbed at site3 are 1.48 Å, 1.47 Å, and 1.51 Å, respectively. Therefore, the adsorbed K+ cation participates in the catalytic process during ORR, leading to an enhancement in catalytic activity.
Conclusion
In summary, we proposed a new “atomic symbiotic-catalyst” system, in which single-atom MNx moieties and local carbon defects formed simultaneously under a specific thermodynamic condition, for achieving the high-efficiency electrocatalytic reactions and batteries synergistically. Marrying the structural precision and preparation accessibility advantages of typical single-atom catalysts and the multi-active-sites synergistic effect of dual-atom catalysts, the symbiotic-catalyst can outperform the performances of conventional single-atom and dual-atom electrocatalysts. In our proof-of-concept study, a MnN4/Cd atomic symbiotic-catalyst coupling MnN4 single-atom sites and abundant sp3-hybridized carbon defects was constructed via a biomass precursor strategy. Notably, the MnN4/Cd symbiotic-catalyst showed a high ORR performance and the derived solid-state ZAB displayed a high peak power density of 76 mW cm−2 with long-time stability under the ultra-low-temperature (−40 °C), which is the top-level performance reported to date. Thorough experimental studies combined with DFT calculations suggested that the adsorbed K+ ion on the sp3-C near MnN4 site could not only tune the electronic state of the active center but also facilitate the weakening of the O−O bond of *OOH intermediate, thus greatly decreasing the ORR overpotential, which resulted in the high performance of low-temperature ZAB.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (22109118, 21890383, 21871159, 22171157, 22109120, 21706196), Key Projects of Zhejiang Natural Science Foundation (Nos. LZ20E010001), National Key R&D Program of China (2018YFA0702003), Science and Technology Key Project of Guangdong Province of China (2020B010188002). The authors thank the BL14 W1 station in Shanghai Synchrotron Radiation Facility (SSRF) for help with characterizations. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.