Earth: An Oxidative Planet with Limited Atom Resources and Rich Chemistry
Graphical Abstract
The depletion of natural resources and the extensive use of oxidative processes by humans violate a number of Earth's planetary boundaries, threatening our very existence. Chemistry is largely responsible for this situation, but it is also central to the solutions. Transitioning to a more “reductive” approach—especially using green hydrogen and sustainable chemistry within the framework of a circular economy—is one of the key solutions.
Abstract
Humanity faces an unprecedented survival challenge: climate change, driven by the depletion of natural resources, excessive waste generation, and deforestation. Six out of nine planetary boundaries have been exceeded, signaling that Earth is far from a safe operating space for humanity. In this Viewpoint Article we explore three critical “atomic-molecular” challenges: Earth's limited atomic resources, its oxidative nature, and very rich chemistry. Addressing these requires a transformation in how we produce and consume, emphasizing sustainable practices aligned with the United Nations’ 17 goals. The advancement of science and technology has extended human life expectancy and improved quality of life. However, to ensure a sustainable future, we must move towards less oxidative chemical processes, incorporate CH4−CO2 redox chemistry into the circular economy, and transition from a linear, fossil fuel-dependent economy to a circular bioeconomy. Reforestation and the recovery of degraded lands are essential, alongside the shift towards green and sustainable chemistry. Earth's dynamic chemistry is governed by the principles of thermodynamics and kinetics, but science alone is insufficient. Achieving global sustainability requires coordinated economic, political, and social decisions that recognize Earth's limited resources and oxidative nature. Together, these efforts will position humanity to meet the challenges of climate change and secure a sustainable future.
General Context. Humanity is facing one of the most significant challenges for its survival since its emergence in the evolutionary tree: climate change. The depletion of natural resources, excessive waste generation, burning of fossil fuels for generating power, transportation and manufacturing goods, deforestation for agriculture or urban development and unbridled consumption are key contributors to the catastrophic effects of climate change.1 Some of the evidence for climate change is summarized in Figure 1a.2 For instance, the increase in global temperatures is resulting in more frequent and severe heatwaves, which not only affect human health and agriculture but also contribute to wildfires. Extreme weather events such as hurricanes, typhoons, and heavy rainfall are becoming more intense and unpredictable, causing widespread flooding, infrastructure damage, and displacement of communities.
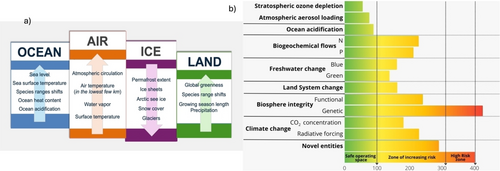
a) Evidence for climate change (extracted from ref.2). The arrows pointing up indicate increases and arrows pointing down decreases. b) Current status of control variables for all nine planetary boundaries. The green zone is the safe operating space (below the boundary). Yellow to red represents the zone of increasing risk. Purple indicates the high-risk zone where interglacial Earth system conditions are transgressed with high confidence (extracted from ref.5). Control variables. 1. Climate change: atmospheric CO2 concentration (ppm) and an total anthropogenic radiative forcing at top-of- atmosphere (W m−2). 2. Change in biosphere integrity: genetic diversity (extitions per million species-years) and functional integrity: measured as energy available to ecosystems that measures the human appropriation of net primary production. 3. Stratospheric ozone depletion: global average of stratospheric O3 concentration. 4. Ocean acidification: carbonate ion concentration, average global surface ocean saturation state with respect to aragonite. 5. Biogeochemical flows: P and N cycles. Phosphate global: P flow from freshwater systems into the ocean; regional: P flow from fertilizers to erodible soils. Nitrogen global: industrial and intentional fixation of N. 6. Land system change: global: area of forested land as the percentage of original forest cover; biome: area of forested land as the percentage of potential forest (% area remaining). 7. Freshwater change: blue water: human induced disturbance of blue water flow and green water: human induced disturbance of water available to plants (% land area with deviations from preindustrial variability). 8. Atmospheric aerosol loading: interhemispheric difference in erosol optical depth. 9. Novel entities: percentage of synthetic chemicals released to the environment without adequate safety testing.
Sea-level rise, driven by the melting of polar ice caps and glaciers as well as the thermal expansion of seawater, threatens coastal cities and small island nations with increased flooding and erosion. Ecosystem disruptions are evident in phenomena such as coral bleaching, where warmer ocean temperatures cause corals to expel the algae they rely on for energy, leading to large-scale die-offs. Additionally, changes in temperature and precipitation patterns are altering the habitats and migration patterns of many species, threatening biodiversity and leading to the extinction of vulnerable species. The shifting climate also impacts agriculture, with some regions experiencing droughts and reduced crop yields while others may face new pests and diseases.3 Six out of nine planetary boundaries have been exceeded, indicating that Earth is far from a safe operating space for humanity (Figure 1b). Ocean acidification is approaching its limit, aerosol loading surpasses regional boundaries, and stratospheric ozone levels have slightly improved. The severity of transgressions in previously overstepped planetary boundaries4 has intensified. Human appropriation of net production, which refers to the consumption of more biological resources than ecosystems can regenerate, has also exceeded sustainable limits. This overuse of resources, crucial for maintaining biosphere functions, poses a significant threat to the long-term stability of ecosystems and the services they provide. Earth system modeling shows that the impacts of climate and land system changes must be viewed systemically due to their interconnectedness.5
These interconnected effects of anthropogenic climate change underscore the urgent need for comprehensive strategies to mitigate and adapt to its impacts. It is estimated, using the ecological footprint measures, that more than four Earth-like planets would be needed to provide the resources for and absorb the waste of the entire global population (over 8 billion)6 if all were to live at the same current standard as in the USA.7
In this Viewpoint Article, we will discuss three significant atomic-molecular challenges facing life on our planet: its limited atomic resources, oxidative nature, and complex chemistry. Additionally, we will explore some of the scientific and technological approaches currently being developed to pave the way for a sustainable society, in alignment with the 17 goals set by the United Nations,8 should humankind decide to survive.9
Limited Atom Resources. Since the formation of Earth over 4.5 billion years ago, its mass has varied due to both accretion and loss of material. Earth gains mass from the continuous accretion of micrometeorites and cosmic dust, while it loses mass primarily through the escape of hydrogen and helium gases into space. Despite these processes, the overall effect is a net loss of material, estimated at approximately 5.5×107 kg per year.10 However, the total mass of heavier elements other than hydrogen and helium experiences relative little change, and these elements are primarily confined to the Earth's crust (see Figure 2). Only 21 of the 118 known elements on Earth have abundance exceeding 100 ppm! Highly strategic elements for today's society needs such as lithium (essential for batteries) and noble metals (electronics and catalysts) have low abundance <100 ppm.11 Earth has a limited number of atoms; the use of some elements may be considered as unsustainable and their current use may create severe both economic and environmental pressures.12 Note that mining activities are unsustainable and abundance limited, and that mining from asteroids or the moon13 is a dream, at least for the near future. At the same time, metals such as cobalt and lithium are essential to sustain the energy transition.14 The amount of ore mined and waste rock removed to produce a refined unit of a precious metal like gold is only in the range of 10−5 to 10−6 of the total material extracted.15
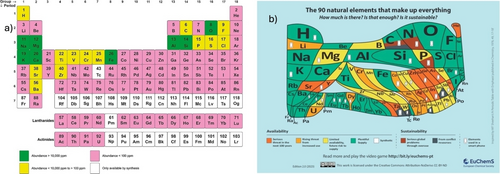
a) Quantitative and b) qualitative representations of atom abundances in the Earth's crust and atmosphere. The data for Figure 2b are available from Periodic Table 7 ref. [21] and the Figure is reproduced with permission from ref. [11]. For a detailed discussion of Table 2b see ref. [22].
Metal recovery and reuse are viable solutions for addressing the challenges of metal use in today's society,16 particularly when employing sustainable technologies.17 This approach offers several benefits such as resource conservation, energy efficiency, waste reduction, “cost”, and reduction of greenhouse gas emissions. Among the various approaches for sustainable technologies, it is worth mentioning the use of electricity to recover metals from electronic waste (e-waste) and industrial wastewater, bio-metallurgy (bioleaching and biosorption), mechanical processing (like shredding, grinding, and separation), and chemical recycling, technologies that are particularly useful for recovering metals from batteries and catalysts. Urban mining, closed-loop recycling and industrial symbiosis are examples of metal recovery and reuse initiatives.17, 18
It is also crucial to develop hybrid materials that mimic the properties of metals and could potentially replace them in various applications.19 These materials combine different elements and compounds to create composites with desirable characteristics such as strength, conductivity, and corrosion resistance, similar to metals used in today's society. Research in materials science is continually advancing, leading to the development of new hybrid materials that meet the demands of various industries while addressing the limitations of limited availability of metals. These materials not only offer potential substitutes for metals but also open new possibilities for innovative applications.20
By investing in and advancing sustainable technologies for metal recovery and reuse, society can reduce its reliance on virgin materials, minimize environmental impacts, and move towards a more circular economy. “The use of the (chemical) elements by the current generation should not restrict the use by future generations.”23 Elemental sustainability ensures that the current use of elements does not restrict future use and promotes their holistic utilization throughout their life cycle.24
In fact, recycling is crucial for sustainability as it conserves natural resources, reduces waste in landfills, and decreases pollution, thus helping to protect the environment for future generations. By recycling, we also save energy and reduce greenhouse gas emissions, contributing to the fight against climate change.25 For example, recycling reduces the need for new plastic made from raw materials, saving energy and carbon in the process. It takes 75 % less energy to make a plastic bottle using recycled plastic compared to newly made plastic.26
It is crucial to emphasize the importance of biodegradable materials, particularly in mitigating the microplastic plague.27 However, it is important to note that, at the end of their life cycle, these materials will still generate CO2.28
Oxidative Planet. The Earth is an oxidative planet where natural oxidation processes, and more recently “artificial” ones introduced by man, play a crucial role in the composition of the atmosphere. It should be noted that life on Earth has always been linked to oxidative processes that generate energy. The Earth's earliest atmosphere lacked oxygen, and the first unicellular organisms were anaerobic. These chemoheterotrophic organisms obtained their vital energy by oxidizing organic compounds to CO2, donating electrons to inorganic compounds such as SO42− to generate H2S as a waste product. However, on a planet abundant in water and bathed in sunlight, the ancestors of cyanobacteria developed the photoenzymatic capability to use H2O as an electron donor to reduce CO2, transforming it into the necessary organic compounds for their vital activity, and producing O2 as a byproduct. The intense activity of these anaerobic photoautotrophic organisms not only modified the composition of the Earth's atmosphere by introducing O2 in increasing concentrations up to the current value of 21 %, but also promoted the evolution of the first eukaryotic organisms to exploit the energetic advantages of aerobic metabolism. Modern aerobic eukaryotic cells originated from the endosymbiosis of anaerobic purple bacteria, which evolved mitochondria as the true oxidative powerhouses of cells where O2 is consumed and energy is generated. Aerobic photosynthetic cells were generated from the endosymbiosis of ancestral cyanobacteria into aerobic eukaryotic cells, evolving into the current chloroplasts of photosynthetic eukaryotic cells (i.e., plants, green algae, etc.), which are true “industrial” factories for CO2 capture and reduction, as well as for O2 production.
In fact, there are several reasons why Earth may be considered an oxidative planet, but as it is inhabited by a vast array of photosynthetic organisms, such as plants, algae, and cyanobacteria. These organisms use sunlight to convert CO2 and water into glucose and oxygen through the process of photosynthesis, contributing to the oxygen levels in the atmosphere. But there is also “important oxidation in biogeochemical cycles” such as the carbon cycle through processes like cellular respiration, whereby organic matter is broken down, releasing CO2 and water while utilizing oxygen.
Life on Earth has always existed in a dynamic equilibrium of immense proportions between living beings that exclusively carry out oxidative processes, meaning the consumption of O2 and the production of CO2, and those that perform reductive processes with CO2 capture and O2 generation (photosynthetic aerobic organisms). All aerobic living beings, i.e., higher animals, plants, microorganisms, etc., produce CO2 and consume O2 for their vital activity. Since primitive humans first mastered fire, oxidative processes that generate CO2 and consume O2 have played a crucial role in human development, driving societal advancement and well-being across history through innovations in transportation, food production, energy, and more. Besides, many of these human developments, which are part of our daily life habits, have been and continue to be based on the destruction of aerobic photosynthetic organisms that capture CO2 and generate O2 (e.g., deforestation, marine pollution, etc.). At this point, it is necessary to underline that the biodegradability of products at the end of their useful life cycle means their metabolization by microorganisms, which represents the transformation of visible and localized wastes into an invisible and delocalized waste such as CO2.
The CO2 production by human respiration (CO2hr) is not an innocent issue, since the production rate of 0.6–1.3 kg CO2/person/day29 represents a global production of 15.3–33.2 t CO2/person for an average lifespan of over 70 years, or an overall production on the planet of 1,750–3,800 Gt CO2/year. Typically, CO2hr is not a net source of atmospheric CO2 because CO2hr emissions are considered as balanced by CO2 uptake through photosynthesis, like a closed loop without no long-term net impact on the carbon balance on a global scale. Therefore, CO2hr is not directly related to the mitigation of climate change, nor is it listed in emissions inventories or observation projects. However, as can be seen in Figure 3, the data reveal how the exponential growth of the human population, mainly during the last century, has been inevitably accompanied by a reduction in the photosynthetic potential of the biosphere, as a consequence of the massive deforestation to satisfy the needs of society (e.g. food, fuels, etc.) within an uncontrolled and unlimited consumption model.30
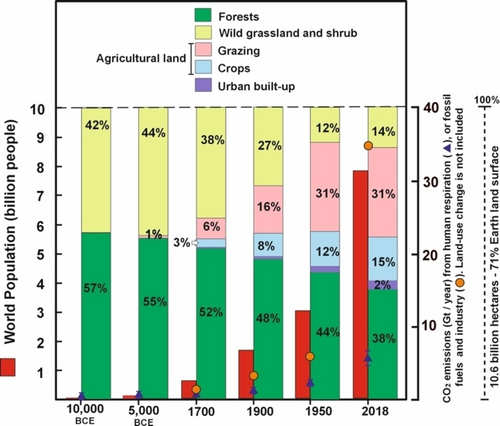
Comparative representation of changes in world population and land uses on the Earth's surface during History. Data obtained from ref. [30].
It should be noted that oxygen is also a fundamental element in many geological and chemical processes on Earth. Besides its essential role in respiration in most organisms, oxygen also plays a central role in the oxidation of organic matter for energy. Oxidation-reduction (redox) reactions involving oxygen are also key in many geochemical processes, such as the weathering of rocks and the formation of minerals, as well as the formation of ozone.
While many chemical processes on Earth involve the consumption of O2 or the generation of CO2, it is important to note that most lead to the production of CO2, while others may not involve carbon at all or may produce or consume different compounds.31 The main processes involve the consumption or generation of CO2. Photosynthesis, conducted by plants, algae, and certain bacteria, is the unique natural process that removes CO2 from the atmosphere. CO2 produced by cellular respiration (CO2cr) and that generated by the combustion of fossil fuels (CO2ff) constitute most of the CO2 present in the atmosphere. Volcanic eruptions also release various gases, including CO2, sulfur dioxide, and water vapor. Weathering and erosion indirectly influence the carbon cycle by affecting the availability of minerals that react with atmospheric CO2 and by decomposing organic matter, which releases CO2 and other gases.
Greenhouse Gas Emissions. CO2 is a common byproduct of many natural and human-induced processes on Earth. The carbon cycle is a natural process that involves the movement of carbon among the Earth's atmosphere, oceans, soil, rocks, and living organisms (Figure 4). This cycle has been integral to Earth's systems for millions of years. However, human activities have significantly disrupted it, leading to increased concentrations of atmospheric CO2. Key human contributions to this disruption include the burning of fossil fuels (e.g. coal, oil, and natural gas) for energy, which releases carbon sequestered in geological formations over millions of years, and the removal of forests for agriculture or urban development, which releases stored carbon from trees and soil (see Figure 4).
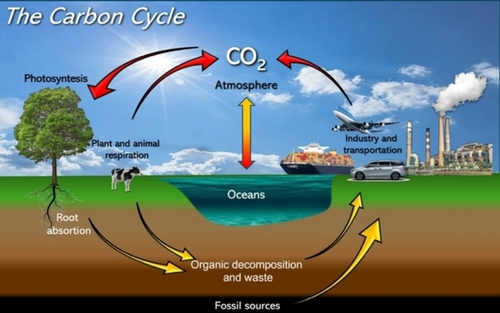
Simplified global carbon cycle (adapted from ref. [2] and (https://edu.rsc.org/resources/carbon-cycle-game/4013431.article) the nature flux involving the exchange between global gross production, respiration and ocean with the atmosphere and the anthropogenic flux (fossil fuels combustion, industrial processes and changes in land-use).
Additionally, soil respiration, influenced by land use, temperature, and moisture changes, releases CO2 as microbes decompose organic matter. The oceans, absorbing about one-fourth of human-emitted CO2, also play a role but face acidification and reduced capacity as carbon sinks.
Natural processes like volcanic eruptions and organic decay contribute to CO2 levels but are minor compared to human activities. The increased CO2 and other greenhouse gases enhance the natural greenhouse effect, trapping more heat and causing global warming. This anthropogenic climate change leads to rising global temperatures, more extreme weather events, sea-level rise, and ecosystem disruptions. It is also crucial to consider that increasing CO2 concentrations correspond with decreasing atmospheric oxygen levels, that is ca. 19 O2 molecules out of every 1 million O2 molecules in the atmosphere each year (Figure 5),32 necessitating careful analysis of geological storage and its global impact.33
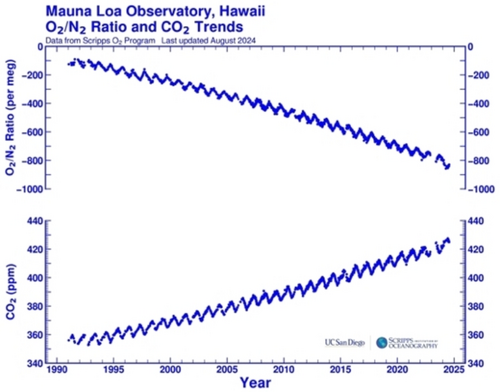
The variation of CO2 and oxygen/nitrogen ratio in the atmosphere from 1991 to August 2024 at the Mauna Loa Observatory, Hawaii. Meg measures oxygen as changes in the O2/N2 ratio of air relative to a reference. It is computed as: δ=((O2/N2) sample—(O2/N2) reference)/(O2/N2) reference, where (O2/N2) sample is the O2/N2 mole ratio of an air sample, and (O2/N2) reference is the O2/N2 mole ratio of the reference, based on tanks of air collected in the mid-1980s and stored at the Scripps Laboratory.34
The role of other greenhouse gases such as methane and dinitrogen oxide should be not underestimated. Methane, an odorless gas from both natural and anthropogenic sources, is a significant contributor to global warming, trapping heat in the atmosphere and aiding in ground-level ozone formation, which is harmful to human health. Although methane's atmospheric lifetime is about 12 years—much shorter than that of CO2—its global-warming potential per unit is more than 20 times that of CO2. Over 20 years, one ton of methane is equivalent to around 85 tons of CO2, and over 100 years, it equals about 28 tons of CO2. Natural wetlands contribute roughly one-third of global methane emissions, while agriculture and fossil fuels each account for 20–25 %. Between 2006 and 2017, methane emissions from agriculture increased by almost 12 % to 227 million tons due to rising red meat consumption, and fossil fuel emissions rose by 17 % to 108 million tons. Livestock farming and oil and gas production are major drivers of increasing methane emissions, with ruminant livestock producing as much methane as the oil and gas industry.35
The remote geographical locations of CH4 sources and the high transport costs often result in a substantial portion of methane being flared at the source. The traditional reforming processes used to convert methane into value-added chemicals are hindered by high energy consumption due to the particularly strong C−H bonds in methane. Conversion of CH4 driven by sustainable energy technologies is not yet optimally designed or economically viable because the conversion technology and sustainable energy transition technology are not well aligned.36 The selective oxidation of methane to methanol remains a significant challenge in catalytic chemistry due to the high energy barrier for initial C−H bond activation and the difficulty in preventing overoxidation to CO2.37 However, recent advancements have been reported in this area, including the use of colloidal metal nanoparticles38 and photoelectrocatalysts based on metal complexes, which open new avenues for utilizing methane as a raw material.39 The enzyme methane monooxygenase (MMO), found in methanotrophic bacteria, is the only known true catalyst, whether industrial or biological, that can convert methane to methanol under ambient conditions with 100 % selectivity.40 While MMO-mediated methane oxidation requires electrons, the subsequent enzymatic conversion of methanol to formaldehyde and formic acid releases electrons, offering a potential for the development of a coupled system. However, the absence of a single enzyme capable of catalyzing the complete oxidation of methane in one step necessitates the use of multiple enzyme cascades, complicating the overall system. Investigating the integration of CO2 biocatalysis with the chemical oxidation of CH4 may provide a promising approach for creating such coupled systems.41
Nitrous oxide (N2O), or “laughing gas,” is the third most emitted greenhouse gas from anthropogenic activities, following CO2 and methane. Its emissions, which have significantly increased in recent decades, are largely from agriculture, biomass burning, and industrial processes like nitric and adipic acid production.42 N2O is almost 300 times more potent as a greenhouse gas than CO2 and has an atmospheric lifetime of approximately 110 years.43 Due to its substantial global warming potential, it is expected to face strict international regulations.44 Currently, industries such as adipic acid production burn N2O in flares, but there is potential to repurpose it as an oxygen source for producing marketable chemicals, with benign dinitrogen as the only by-product.45 Particularly it can be used in photocatalytic oxygen transfer agent for various applications.46
Anthropogenic CO2. Human activities, particularly the burning of fossil fuels and land-use changes, have significantly altered the carbon cycle, leading to an imbalance in the natural processes that regulate atmospheric CO2 levels. This disruption is a major driver of contemporary climate change and its associated impacts. Indeed, in 2021, the total concentration of greenhouse gases and other forcing agents, including cooling aerosols, reached 472 parts per million CO2 equivalents, a level that the International Panel on Climate Change warns should not be exceeded to limit global temperature rise to 1.5 °C above pre-industrial levels (Figure 6) with a 67 % likelihood and no temperature overshoot, while allowing for a temperature overshoot could push this peak before 2026, and concentrations leading to a 2 °C increase by 2100 could be exceeded before 2032.47
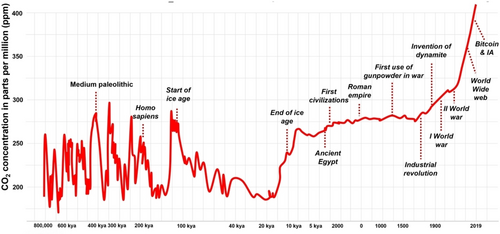
Variation of CO2 concentration in the Earth's atmosphere (kya=1,000 years ago, graphic adapted from [48]).
The total CO2 emissions in 2022 exceeded 40 billion metric tons78 and the major contributions are from fossil fuels (around 10 %) and land-use (around 90 %). The leading CO2 emitting countries are China (accounting for approximately 28 % of global CO2 emissions), USA (around 15 %) European Union (approximately 8 %), India (approximately 7 %), and Russia. The global average per capita CO2 emissions (2020) were approximately 4.5 metric tons in China (7.8 metric tons per capita), USA (16.5 metric tons per capita), European Union (6.3 metric tons per capita), and India (2.7 metric tons per capita) with the largest contributions.49
The CO2 emissions that represent around 76 % of the global greenhouse gas emissions (Figure 7a) vary by sector from 73 % of global CO2 emissions in the energy sector (including electricity and heat production, transportation, and industrial processes), land-use changes and forestry with approximately 17 %, to 9 % from industrial processes (Figure 7b). Also, approximately a quarter of global industrial CO2 emissions come from sources that are hard to eliminate with existing policies and infrastructure.
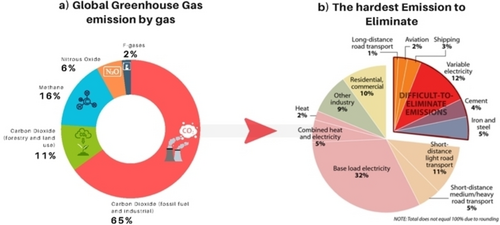
a) Global Greenhouse gas emissions by gas and b) the hardest emissions to eliminate (graphics adapted from ref. [54]).
Another significant source of anthropogenic CO2 emissions is the Haber–Bosch process, used to produce ammonium as an active and useful nutrient for plant growth. Nitrogen (N) is an essential atom for many biomolecules (e.g., proteins, nucleic acids), and although molecular nitrogen (N2) constitutes 78 % of the atmosphere, its triple bond (ΔG′°=930 kJ/mol) makes it a quasi-inert molecule. The biocatalytic transformation of N2 into ammonia by the nitrogenase enzyme complex in certain bacteria (e.g., Azotobacter) is not only a marvel of catalysis but has also been the bottleneck for the development and expansion of life on Earth, as nitrogen is a limiting nutrient for crop and food production. In fact, global nitrogen fixation contributes 413 Tg of reactive nitrogen to terrestrial and marine ecosystems annually of which anthropogenic activities are responsible for half, 210 Tg N.50 With the industrialization of the Haber–Bosch process to produce ammonia from N2 at the beginning of the 20th century, artificial fertilizers became available, leading to an explosion in agricultural production and facilitating access to cheaper food, which has directly impacted overpopulation. It is estimated that only 52 % of the current world population could survive without reactive nitrogen fertilizers derived from the Haber–Bosch process.51
However, the Haber–Bosch process is currently one of the largest global energy consumers, accounting for 1 % of all energy consumption worldwide. It is also a significant greenhouse gas emitter, responsible for 1.2 % of global anthropogenic CO2 emissions. Approximately 1.9 metric tons of fossil CO2 are released per metric ton of ammonia produced, primarily using natural gas to obtain H2, N2, and energy.52 Therefore, developing alternative sustainable processes such as those based on biological methods or photocatalysis is necessary.53
Although biomimetic nitrogen fixation would be ideal, little real progress has been made in 60 years. A much better solution to the problem of producing ammonia would be to decarbonize the Haber–Bosch process by using green hydrogen instead of hydrogen produced from methane. This would reduce CO2 emissions from the process by 90 %. More could be done by using non fossil fuel heating.55 The concentration of CO2 in waste gas from industrial processes can vary from 5–6 % in gas-based power plants to 96 % in bioethanol plants (Figure 8).
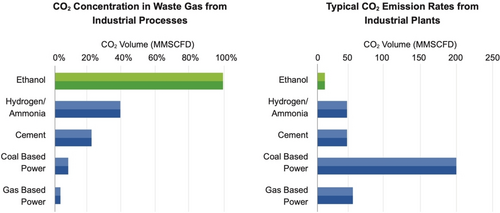
Biogenic and anthropogenic CO2 sources (MMSCFD= Million standard cubic feet per day (1 MMSCFD= 1180 m3/h), extracted from ref.[56]).
Although the CO2 emission rates are lower in bioethanol plants, its concentration makes it logistically and economic suitable for direct capture and bio(chemical) transformation into, for example methanol or formic acid (see below). At first glance, this appears to be a typical case where using CO2 from bioethanol plants for geological storage makes little environmental or economic sense, as the cost of capturing CO2 directly from the air is approaching economically viability.57
On the Capture and Recovery of the Anthropogenic CO2. The use of CO2 depends heavily on the capture technologies such as direct air capture, aqueous amine-based capture, membrane separation, chemical looping, cryogenic carbon capture and carbon capture using nanotechnology.58 While direct air capture and carbon capture at power plants are the most widely used technologies,59 there are promising experimental technologies like chemical looping and nanotechnology.60 It's crucial to stress that scientific and technological efforts are ongoing worldwide to reduce CO2 emissions and mitigate climate change. These efforts involve transitioning to renewable energy sources, increasing energy efficiency, reforestation, and adopting sustainable practices in various sectors.61 Expanding forest areas and prolonging the age of rotation, particularly in exotic tree plantations, have been suggested as significant approaches to maximize carbon capture and minimize the adverse impacts of global climate change, as forest ecosystems can accumulate substantial amounts of carbon. The average carbon content in forests, especially in protected areas, is 105.72 Mg-C/ha, with a carbon absorption rate of 1.03 Mg-C/ha/yr. Since the improvement of carbon capture depends on volume growth, sustainable forest management must be established to maximize output and promote the long-term use of soil fertility.62 The bioeconomy, which utilizes renewable forms of biogenic carbon from agriculture, forestry, and aquaculture for conversion into food, feed, fiber, polymers, bioenergy, and other bioproducts, is central to the key solution for moving away from fossil fuels and other non-renewable carbon sources.63
Most importantly, as already stressed, CO2 should be incorporated as a raw material and become a major part of the circular economy of the chemical industry sector (Figure 9).64
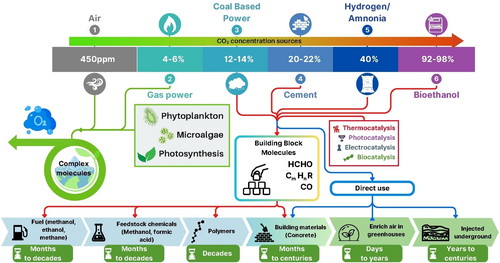
Examples of technologies for reusing CO2 as raw material (extracted and adapted from ref. [65].
There are several technological approaches that can be used to store CO2 for months to decades.65 However, the most sustainable processes involve the reduction of CO2 to chemical commodities and certainly involves the use of catalysis66 following the green chemistry principles.61a Geological storage may be helpful for a transition period but in a long run it involves the reduction of oxygen in the atmosphere (for each atom of carbon stored, two atoms of oxygen are removed from air!) and as already stated, the effect of such reduction should be considered.33a
The catalytic reduction of CO2 by thermo-, bio-, photo- and electrocatalytic processes is the center of technological solutions that allows the use of CO2 as raw material for carbon capture and utilization.67
There are several approaches that can disclosed for the hydrogenation of CO2. For example, over a decade ago the Methanol Economy concept68 was proposed, which was put into practice with the industrial implementation of CO2 reduction to methanol.69
Formic acid for a hydrogen battery70 (Figure 10) is another alternative because it can also be produced from the oxidation of waste organic materials such as water-soluble and non-soluble biomass. The process, depending on the substrate, can generate up to one kilogram of bio-based formic acid per kilogram of dry matter.71
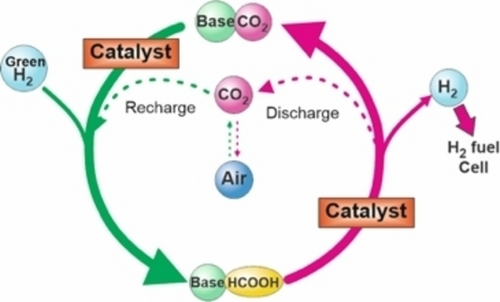
The processes involving the use of formic acid in a hydrogen carrier (adapted from ref. [70b]).
The catalytic hydrogenation of CO2 to free formic acid is thermodynamically unfavorable, and bases are typically required to overcome these barriers, resulting in the production of waste and necessitating the post-processing of formate salts.72 The use of buffers can help mitigate these limitations,73 or alternatively, CO2 hydrogenation can be performed in a biphasic reaction system using aqueous solutions of amino acids as the product phase. The resulting formic acid/amino acid solutions could be used directly, or after further processing, in agricultural applications without the need for energy-intensive and costly isolation of pure formic acid.74
Most of the technological solutions involve the use of hydrogen that must be produced in a green and sustainable manner employing green energies.75 The production of green hydrogen (Figure 11) is a rapidly evolving field with significant potential to contribute to global decarbonization efforts. Green hydrogen is produced through the electrolysis of water using renewable energy sources such as wind, solar, and hydropower, resulting in zero carbon emissions.76 Recent advancements in this area have focused on improving the efficiency and cost-effectiveness of electrolysis technologies.77 Challenges include the high initial costs and the need for large-scale infrastructure, storage and transportation to support widespread adoption. However, the technical, economic, and logistical challenges associated with hydrogen production, storage, transportation, distribution, and utilization remain significant obstacles to achieving net-zero emissions.
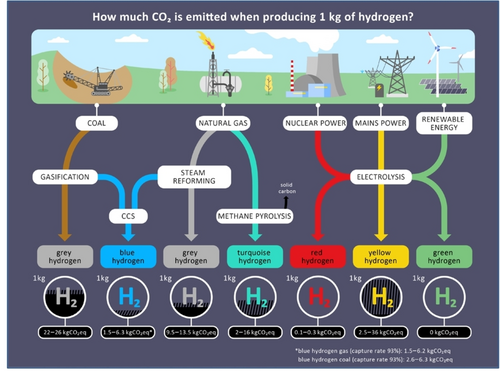
Hydrogen production technologies and associated emissions in kg CO2-equivalent for 1 kg of hydrogen (Source: Energy Systems of the Future (ESYS), illustration by Joseph & Sebastian78).
Nevertheless, a recent report in the UK suggests that green hydrogen could soon become an viable option.79 Currently, about 95 % of global hydrogen production relies on fossil fuels, resulting in “grey hydrogen” that contributes around 2 % of global carbon emissions.76
For hydrogen to support a net-zero system, it must be produced as “blue hydrogen” (where CO2 generated is captured and stored) or “green hydrogen” (produced using renewable energy for water electrolysis). However, the deployment of carbon capture, utilization, and storage (CCUS) technology is currently insufficient to significantly reduce emissions on a large scale, and the necessary economic and commercial conditions for widespread CCUS adoption have not been established.80 As a result, relying on blue hydrogen for substantial decarbonization in the near to medium term is impractical. Additionally, large-scale production of green hydrogen via electrolysis and solar photovoltaics faces several challenges.81
Nevertheless, there has been a veritable explosion of activity targeting the development of direct solar-to-fuels devices, in the hope that these will eventually become more efficient than indirect methods. Such direct solar-to-fuels devices can be considered to perform “artificial photosynthesis” (Figure 12), whereby the energy of sunlight is captured and stored in the form of chemical bonds in a product “solar fuel” (hydrogen, hydrocarbons and other chemicals).82

Natural photosynthesis and artificial photosynthesis (water splitting and reverse (semi)combustion).
Artificial photosynthesis is a giant scientific and technological challenge but it has already show promising results such as the pilot plant for the hydrogenation of CO2 to produce materials for plastic.83 Although the most efficient solar hydrogen production Schemes, which couple solar cells with electrolysis systems, achieve solar-to-hydrogen (STH) energy conversion efficiencies of up to 30 % at a laboratory scale, photocatalytic water splitting attains significantly lower conversion efficiencies of around 1 %.84 However, photocatalytic systems are much simpler, cheaper, and more amenable to scale-up, provided that the moist, stoichiometric hydrogen and oxygen product mixture can be safely managed and the hydrogen effectively recovered.82b
Despite the inefficiency and overall energy-negative nature of hydrogen production via photocatalytic water splitting, recent reports have demonstrated the safe operation of a 100 m2 array of panel reactors over several months. This setup featured autonomous hydrogen recovery from the moist gas product mixture using a commercial polyimide membrane, achieving a maximum STH efficiency of 0.76 %. These findings illustrate that safe, large-scale photocatalytic water splitting, along with gas collection and separation, is feasible.85 Moreover, REPSOL has launched a pilot plant based on the photo-electrocatalysis of water to generate hydrogen, paving the way for the installation of a demonstration industrial plant.86
There are various technologies that can use CO2 as a raw material in different Technology Readiness Levels (from TRL-1 to 9) such as generating methanol from the reduction of CO2 or the production of CO2-based polycarbonates that are already commercially available (Figure 13).
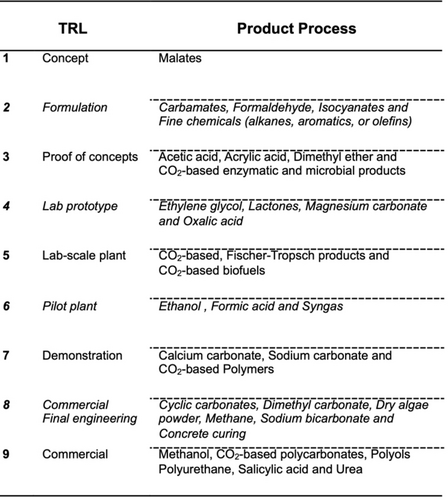
Current state of different technologies that use CO2 as a raw material. Adapted from ref. [87].
Most of these technologies employs relatively pure CO2 as starting material, and the availability of this gas highly depends on the process of capture (see above). However, a more efficient approach involves carbon capture, utilization, and storage (CCUS)67b, 80, 88—mainly chemisorbed CO2-74 in the form of products such as methanol and formic acid i.e. direct carbon capture and utilization (Figure 14).57b, 89
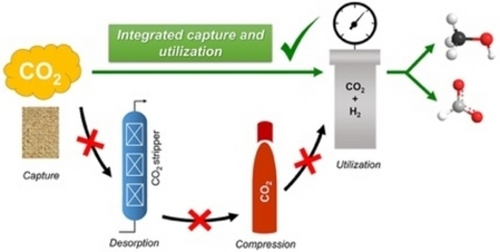
Example of an integrated process that may be used for the direct conversion of CO2 into formic acid or methanol. Reproduced with permission from ref. [90]. Copyright 2019, American Chemical Society.
CCUS technology is more suitable for applications that capture CO2 emissions from stationary sources like factories and manufacturing plants, but it is difficult to capture emissions from mobile sources like vehicles. Various technologies are available for capturing CO2 from stationary sources, and the field is continually evolving with new methods. CO2 has many applications, making its utilization economically appealing to manufacturers such as algae cultivation, chemical synthesis, carbon mineralization, and enhanced oil recovery.65
It is important to note that only around 45 commercial facilities are currently operational, applying CCUS technologies to industrial processes, fuel transformation, and power generation. Although the deployment of CCUS has lagged behind expectations in the past, recent years have seen significant momentum, with over 700 projects at various stages of development across the CCUS value chain (Figure 15).
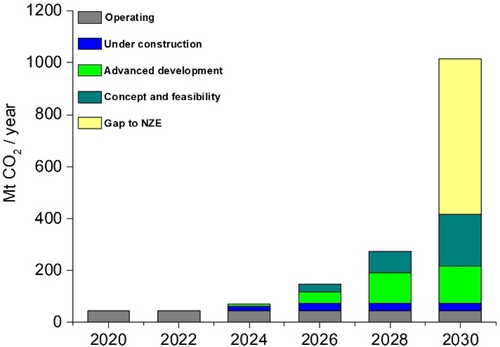
Capacity of current and planned large-scale CO2 capture projects vs. the NetZero Scenario,2020–2030. Extracted from ref. [91].
In 2023, the announced CO2 capture capacity for 2030 increased by 35 %, while announced storage capacity surged by 70 %. This brings the potential CO2 capture in 2030 to approximately 435 million tonnes (Mt) per year, which represents only 1.16 % of the 37.4 billion tonnes of CO2 emitted in 2023. Additionally, the announced storage capacity has risen to only about 615 Mt of CO2 per year. While this progress is somehow encouraging, these Figures still represent only around 40 % (for capture) and 60 % (for storage) of the 1 gigatonne (Gt) of CO2 per year required in the Net Zero Emissions by 2050 (NZE) Scenario (Figure 15).91 It is clear that reducing the remaining 98 % of CO2 emissions must involve integrated approaches, such as transitioning towards a “bio-based economy”, substantially reducing the use of fossil fuels for transportation, recovering degraded areas, reforestation, and fundamentally changing our way of living.
The knowledge and technological advancements necessary to incorporate CO2 into a circular economy are already available albeit may be not yet ideal. The key question is whether and when we will choose to effectively implement them. The argument that green and sustainable technologies are inherently more expensive than conventional ones does not hold in a truly competitive market. In fact, fossil fuel industries benefit from significant subsidies, receiving over 14 million USD per minute in 2023, according to the International Monetary Fund.92 The apparent short-term economic costs of implementing sustainable technologies pale in comparison to the long-term catastrophic destruction already caused by climate change.
Lessons and Perspectives. The advancement of science and technology over the past two centuries has significantly improved the quality of life for humanity. Life expectancy has risen from less than 35 years to over 70 years, thanks to innovative approaches to producing goods and providing services. Undoubtedly, basic science provides the necessary knowledge for technological advancements that address today's challenges and will position us for sustainable development, ensuring the future of humankind on Earth. Indeed, science and technology are already offering solutions. We must move towards a less oxidative planet by incorporating more reduction in our chemical processes and integrating CH4−CO2 redox chemistry and its limited atom resources into the circular economy. Transitioning from the fossil resource-dependent linear economic model of “take-make-waste” to a circular bioeconomy is inevitable as well as recovery and reuse of the limited atom resources. And it is evident that one of the key solutions is reforestation and the recuperation of degraded land areas. The production of goods must shift towards green and sustainable chemistry. Earth is a planet with rich and dynamic chemistry, where continuous processes of bond formation and bond breaking are governed by the inviolable principles of thermodynamics and the inevitable laws of kinetics. However, science is only a part—albeit a very important part—of the solution. International agreements on “CO2 Emission Rights” are clearly insufficient rules to push against climate change, and too ambiguous for individual citizens committed to this fight. Introducing new concepts, such as the “O2 Production Duty”, may be a crucial stimulus, both individually and collectively, to pull towards a reduction in atmospheric CO2 levels by encouraging reforestation and the preservation of natural spaces.
“The high preexisting levels of belief and policy support, alongside the small effect sizes observed, raise critical questions about the practical capacity to facilitate bottom-up change at a global level, suggesting that top-down change might need to be prioritized to achieve the emissions reduction necessary to stay within safe planetary limits for human civilization.”93
Economic, political, and social policies are also essential to change our way of living, incorporating the Earth's limited resources and oxidative nature, and achieving a global coordinated effort. Human security, defined as “freedom from want, fear, and the ability to live with dignity,” addresses threats such as health, food security, economic stability, and environmental challenges, all while focusing on individuals. Chemistry plays a vital role in tackling these issues, though its contributions to human security have not been widely recognized.94 To integrate chemistry into the human security framework, it is necessary to highlight its key contributions and adapt to global challenges by enhancing methods and expanding expertise. By positioning chemistry at the center of human security, it can become more impactful, requiring greater engagement at the intersection of science, society, and policy.
Disclaimer
The opinions expressed in this publication are the view of the author(s) and do not necessarily reflect the opinions or views of Angewandte Chemie International Edition/Angewandte Chemie, the Publisher, the GDCh, or the affiliated editors
Acknowledgments
This work has been partially supported by MICINN-FEDERAEI 10.13039/501100011033 (PID2021-124695OB-C21/C22 and PDC2022-133313-C21/C22), MICINN—European Union Next Generation EU-PRTR (TED2021-129626B-C21/C22) and Fundación SENECA CARM (21884/PI/22) grants. We extend our gratitude to FAPERGS, CNPq, CAPES, FINEP, the Humboldt Foundation, the Beatriz Galindo Foundation, and Petrobras for their financial support over the past decades. Special thanks to Dr. G. Chacon and Dr. Wellington D. Gonçalves for their assistance in preparing the drawings presented in the figures.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Biographical Information
Jairton Dupont, born in Rio Grande do Sul, Brazil, earned his PhD in Chemistry from Université Louis Pasteur, Strasbourg (1988). After postdoctoral work at Oxford, he joined the Federal University of Rio Grande do Sul in 1990, becoming a Professor in 2008. He was EPSRC/GSK Chair at the University of Nottingham (2014–2017) and later returned to Porto Alegre. With over 400 publications, Dupont is a Fellow of RSC, ABC, and TWAS. His research focuses on ionic liquids, catalysis, nanomaterials, and alternative energies.
Biographical Information
Prof. Pedro Lozano, born in 1961 in Ceutí (Spain), earned his Chemistry degree (1984) and PhD (1988) from the University of Murcia. After postdoctoral training in France (1990–1991), he returned to Murcia, becoming a Full Professor in 2004. He served as Vice Dean (1996–2014) and Dean (2014–2022) of the Faculty of Chemistry. His research focuses on enzymatic technology in non-conventional media, with over 140 publications. He received the IQS-European Award (2003), RSC Fellowship (2017), and ANQUE National Award (2020).