Steering the Selectivity of CORR from Acetate to Ethanol via Tailoring the Thermodynamic Activity of Water
Graphical Abstract
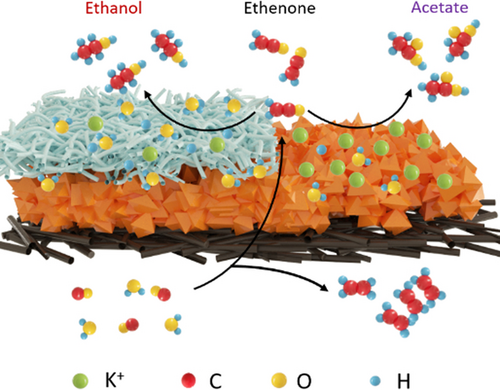
A universal approach was developed to tune the selectivity of Cu-based catalysts in CORR from acetate to ethanol by tailoring the thermodynamic activity of water. The faradaic efficiencies of ethanol and alcohol can reach 42.5 % and 55.1 % at 700 mA cm−2, respectively. The ability to control the selectivity of CORR opens up new possibilities for synthesizing a wider range of valuable chemical products from CO2.
Abstract
The electrochemical conversion of carbon monoxide (CO) into oxygenated C2+ products at high rates and selectivity offers a promising approach for the two-step conversion of carbon dioxide (CO2). However, a major drawback of the CO electrochemical reduction in alkaline electrolyte is the preference for the acetate pathway over the more valuable ethanol pathway. Recent research has shed light on the significant impact of thermodynamic water activity on the electrochemical CO2 reduction reaction pathways, but less is understood for the electrochemical reduction of CO. In this study, we investigated how the water activity at the electrified interface can be enhanced to adjust the selectivity between acetate and ethanol. We employed an ionomer modifier to lower the local concentration of alkali ions (via Donnan exclusion), successfully enhancing ethanol production while suppressing acetate formation. We observed a remarkable improvement in the Faradaic efficiency of ethanol and alcohol (i. e. ethanol, propanol etc), which reached 42.5 % and 55.1 %, respectively, at a current density of 700 mA cm−2. The partial current densities of ethanol and alcohol reached 698 and 942 mA cm−2 at 2000 mA cm−2. Furthermore, we achieved a 3.7-fold increase in the ethanol/acetate ratio, providing clear evidence of our successful modulation of product selectivity.
Conflict of Interests
The authors declare no competing financial interests.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.