Growth Kinetics of Graphene on Cu(111) Foils from Methane, Ethyne, Ethylene, and Ethane
Graphical Abstract
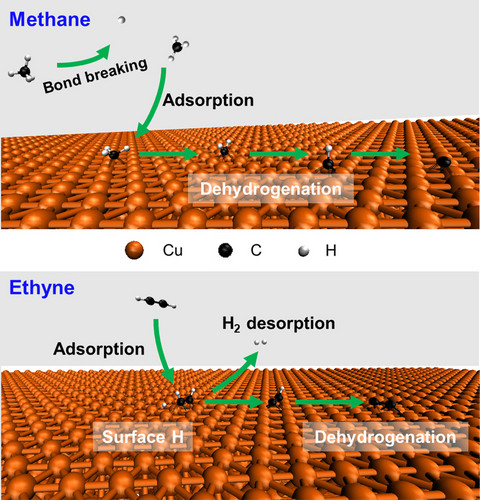
We obtained the activation enthalpies of graphene island growth on Cu(111) foils using H2 and: CH4, and C2H2, C2H4, and C2H6, respectively. The rate determining step (RDS) for CH4 is its gas phase decomposition to H and CH3. The RDS for C2H2, C2H4, and C2H6 is dehydrogenation on the Cu(111) surface yielding adsorbed C2H1, C2H3, and C2H5, respectively.
Abstract
Chemical vapor deposition of carbon precursors on Cu-based substrates at temperatures exceeding 1000 °C is currently a typical route for the scalable synthesis of large-area high-quality single-layer graphene (SLG) films. Using molecules with higher activities than CH4 may afford lower growth temperatures that might yield fold- and wrinkle-free graphene. The kinetics of growth of graphene using hydrocarbons other than CH4 are of interest to the scientific and industrial communities. We measured the growth rates of graphene islands on Cu(111) foils by using C2H2, C2H4, C2H6 and CH4, respectively (each mixed with H2). From such kinetics data we obtain the activation enthalpy (ΔH≠) of graphene growth as shown in parentheses (C2H2 (0.93±0.09 eV); C2H4 (2.05±0.19 eV); C2H6 (2.50±0.11 eV); CH4 (4.59±0.26 eV)); C2Hy (y=2, 4, 6) show similar growth behavior but CH4 is different. Computational fluid dynamics and density functional theory simulations suggest that C2Hy differs from CH4 due to different values of adsorption energy and the lifetime of relevant carbon precursors on the Cu(111) surface. Combining experimental and simulation results, we find that the rate determining step (RDS) is the dissociation of the first C−H bond of CH4 molecules in the gas phase, while the RDS using C2Hy is the first dehydrogenation of adsorbed C2Hy that happens with assistance of H atoms adsorbed on the Cu(111) surface. By using C2H2 as the carbon precursor, high-quality single-crystal adlayer-free SLG films are achieved on Cu(111) foils at 900 °C.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.