Unlocking the Power of Human Ferritin: Enhanced Drug Delivery of Aurothiomalate in A2780 Ovarian Cancer Cells
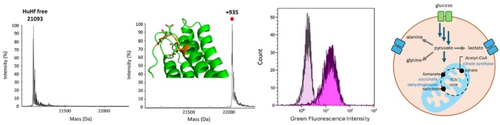
Graphical Abstract
Aurothiomalate (AuTM), a potential anticancer drug, is here conjugated to human H ferritin (HuHf), which specifically targets tumor cells via binding transferrin receptor 1 (TfR1). The formation of a highly reproducible HuHf@AuTM bioconjugate is verified by ESI MS and other biophysical and computational methods. NMR metabolomics and antiproliferative tests point out that HuHf@AuTM is far more active than free AuTM.
Abstract
Aurothiomalate (AuTM) is an FDA-approved antiarthritic gold drug with unique anticancer properties. To enhance its anticancer activity, we prepared a bioconjugate with human apoferritin (HuHf) by attaching some AuTM moieties to surface protein residues. The reaction of apoferritin with excess AuTM yielded a single adduct, that was characterized by ESI MS and ICP-OES analysis, using three mutant ferritins and trypsinization experiments. The adduct contains ~3 gold atoms per ferritin subunit, arranged in a small cluster bound to Cys90 and Cys102. MD simulations provided a plausible structural model for the cluster. The adduct was evaluated for its pharmacological properties and was found to be significantly more cytotoxic than free AuTM against A2780 cancer cells mainly due to higher gold uptake. NMR-metabolomics showed that AuTM bound to HuHf and free AuTM induced qualitatively similar changes in treated cancer cells, indicating that the effects on cell metabolism are approximately the same, in agreement with independent biochemical experiments. In conclusion, we have demonstrated here that a molecularly precise bioconjugate formed between AuTM and HuHf exhibits anticancer properties far superior to the free drug, while retaining its key mechanistic features. Evidence is provided that human ferritin can serve as an excellent carrier for this metallodrug.
Introduction
Aurothiomalate, AuTM, (Figure 1A) is an injectable thiolate gold(I) drug approved by the FDA in 1969 for the treatment of rheumatoid arthritis.1 AuTM is available as a powder containing polymeric species in which the gold(I) ions are linearly coordinated to sulfur ligands;2 spectroscopic and structural evidence suggests that the oligomeric chain adopts a helical conformation.2-5 More recently, AuTM was found to have promising anticancer properties and was eventually approved for clinical trials in cancer through drug repurposing (ClinicalTrials.gov ID: NCT00575393, NCT01383668). Subsequent cellular and molecular pharmacology studies identified protein kinase C iota as the primary biomolecular target for the anticancer activity of AuTM, this being an innovative and peculiar mechanistic feature.6 Indeed, protein kinase C (PKC) isoenzymes play key regulatory roles in many cellular functions, including the control of cell proliferation and motility and the survival of human cancer cells.7 Atypical protein kinase Cι (PKCι) has also been implicated in the oncogenic growth of a variety of cancer cells, including non-small cell lung cancer (NSCLC), ovarian cancer, pancreatic cancer and colon cancer.8 Mechanistically, AuTM was found to bind directly to a specific cysteine residue unique to the PKCι PB1 domain, thereby inhibiting Par6 (Partitioning defective 6 homolog alpha protein) binding.6
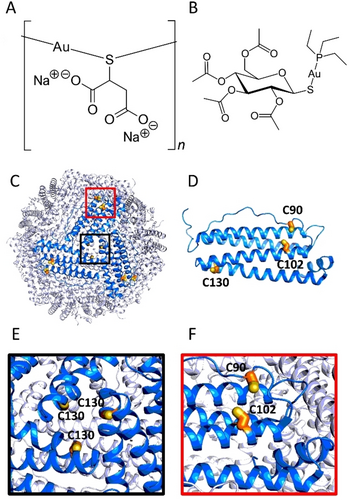
Relevant structural features of gold(I) drugs and HuHf. A) Chemical structure of AuTM. B) Chemical structure of AF. C–F) Structure of HuHf (drawn using Pymol and the coordinates from PDB ID: 4Y08): C) outside view of the 24-mer nanocage; the position of Cys130 at the entrance of one of the eight 3-fold symmetry channels is boxed in black; the position of the two solvent-exposed Cys90 and Cys102 on one of the 24 subunits is boxed in red; D) cartoon representation of the H subunit structure, with cysteine residues highlighted as yellow sticks; E) close-up view of the entrance of a 3-fold channel showing the three symmetry-related Cys130; F) close-up view of the two solvent-exposed Cys90 and Cys102.
Comparative studies with the reference gold(I) drug auranofin (AF) (Figure 1B) showed that AuTM is much less cytotoxic and induces less oxidative stress and less mitochondrial damage than AF.9 These results also suggested that AuTM is less prone to enter cancer cells and that gold uptake is much lower than for AF. Recent work in our laboratory on the formation, biophysical characterization, and biological evaluation of bioconjugates between human ferritin and AF demonstrated that ferritin bioconjugates have superior cellular activity than free AF.10 Accordingly, in this work, we prepared ferritin adducts of AuTM with the aim of improving its anticancer profile through a greater and more selective cellular uptake. Human ferritin is a nano-sized cage protein composed of 24 self-assembling subunits (Figure 1C–F);11 the cytoplasmic protein is typically a heteropolymer with a variable composition of H and L chains. The choice of recombinant homo-polymeric human H-ferritin (HuHf) as a nanocarrier is dictated by the growing evidence that this nanocage protein can be internalized via an endocytic process mediated by the transferrin receptor 1 (TfR1).12, 13 The overexpression of TfR1 in cancer cells, the low immunogenicity of human ferritin14, 15 and the possibility of obtaining bioconjugates with small therapeutic molecules by encapsulation in the internal cavity16-20 or by decoration of the external cage surface21 make ferritin an optimal drug carrier for the targeted delivery of anticancer agents.
We have recently shown how Cys90 and Cys102 (Figure 1F), located on the outer surface of each H subunit can provide a high affinity anchoring point for AF.10 By analogy, we decided to prepare and characterize bioconjugates between AuTM and HuHf obtained by the direct reaction between the metallodrug and the ferritin nanocage under near-physiological solution conditions. This reaction typically results in metal coordination to protein surface residues without protein degradation. The basic idea is that when the protein is exposed to a large molar excess of the metallodrug of interest, the surface binding sites—most likely corresponding to solvent-exposed free cysteines—are fully occupied by gold(I) through the formation of strong gold-thiolate coordination bonds. The unreacted metallodrug can then be removed by repeated washing, while the gold-sulfur coordination bonds formed are sufficiently stable to be retained. Interestingly, we found here that when ferritin is mixed with an excess of AuTM only a single adduct is obtained with a rough stoichiometry of 3 gold atoms per ferritin subunit. The obtained adduct was thoroughly characterized by a variety of biophysical methods and a consistent structural model was proposed on the ground of MD simulations. Subsequently, its anticancer activity was evaluated in vitro against A2780 cancer cells and the effects on cancer cell metabolism were studied. Overall, the results obtained for this bioconjugate are very encouraging, suggesting a net improvement in the anticancer properties compared to the free gold drug, while maintaining the characteristic effects on the cellular metabolome.
Results and Discussion
Reaction of Native HuHf with AuTM and Biophysical Characterization of the HuHf@AuTM Adduct
Intact HuHf was dissolved in PBS and then reacted by simple incubation with increasing amounts of AuTM (to achieve AuTM:nanocage molar ratios of 24 : 1, 72 : 1, 120 : 1, 240 : 1, 480 : 1 and 720 : 1). The interaction between apo-HuHf and AuTM in solution was investigated by ESI MS measurements. The spectra of each sample were recorded after 3 hours of incubation followed by extensive dialysis. The ESI MS spectrum of native HuHf shows an intense peak with a molecular mass of 21093 Da, which can be readily assigned to the monomeric species of the protein; indeed, it is well known that the ferritin nanocage is broken down into individual subunits during the ESI process.22 The complete titration profile is shown in Figure 2. Interestingly, the addition of increasing amounts of AuTM induces the progressive appearance of a new peak at 22057 Da corresponding to a protein adduct carrying a metallic fragment of mass 935 Da per subunit. It is noteworthy that, as the titration proceeds, the peak of the adduct continuously increases in intensity, while the peak of the native apo-subunit decreases until it disappears.
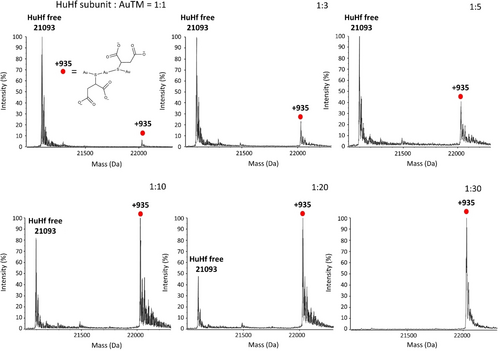
AuTM binding to wild type HuHf. Deconvoluted ESI MS spectra of HuHf at increasing amount of AuTM. The AuTM: nanocage ratio were: 24 : 1, 72 : 1, 120 : 1, 240 : 1, 480 : 1 and 720 : 1 that correspond to the protein subunit:AuTM ratios indicated in the corresponding panels. The +935 Da fragment corresponds to the formula Au3TM2Na2; the structural formula of the Au3TM2 species is reported in the first panel.
We interpret this spectral behavior as the progressive and complete conversion of the native protein into a single type of HuHf@AuTM adduct. The binding process appears to be highly cooperative: indeed, no other adducts showing different mass increases were detected during the titration. We then attempted to understand the nature of the protein bound fragment of mass 935 Da. This increase in mass is consistent with the binding of a metallic fragment with the general formula Au3TM2Na2 (theoretical mass of 935.1619 Da). The characteristic profile of the ESI MS titration inspired the experimental procedure to prepare the HuHf@AuTM bioconjugate. To this end, the HuHf nanocage was dissolved in PBS buffer and then reacted with a large molar excess of AuTM (at an AuTM:HuHf nanocage ratio of 720 : 1, which corresponds to an AuTM:subunit ratio of 30 : 1), for 3 hours at room temperature (RT), pH 7.4, followed by repeated dialysis against buffer. The gold content per cage was quantified by ICP measurements. An aliquot of the sample was subjected to buffer exchange to make the sample suitable for ESI MS analysis. The resulting ESI MS spectrum is fully consistent with the final spectrum of the above titration, demonstrating the complete conversion of apoferritin into the bioconjugate. No signal for apo-HuHf is detected, indicating that the bioconjugate is stable to extensive dialysis and no gold release occurs. ICP results indicate an average of ~70 gold atoms per HuHf cage (i.e., ~2.9 gold atoms per subunit). Notably, this experimental procedure was highly reproducible and constituted the standard protocol for the preparation of the HuHf@AuTM bioconjugate throughout this study.
ESI MS studies previously performed on a number of model proteins suggest that gold compounds preferentially bind proteins at the level of free cysteines.23-26 The exclusive binding of AF to the free cysteines of human ferritin (i.e, Cys90, Cys 102 and Cys130, Figure 1D) was clearly documented in our previous study.10 We thus hypothesized that the binding of AuTM to apoferritin might also occur at the level of the free cysteines of the protein. To validate this hypothesis, some mutants of HuHf were used in which the three free cysteines of the protein (Figure 1D–F) are replaced by Ala residues. Specifically, the following HuHf mutants were used for AuTM binding studies: the single mutants C90A and C130A, and the double mutant C90AC102A. As in the case of wild type apoferritin, these mutant ferritins were challenged with a saturating amount of AuTM for 3 h at RT and the resulting ESI MS spectra were recorded after extensive dialysis (Figure 3). The corresponding titrations are shown in Figure S1 and Figure S2. The ESI MS spectra highlight remarkable differences in the respective reaction profiles. In fact, the C90A and C90AC102A mutants show an almost complete or even complete loss of the ability to bind gold, clearly confirming the pivotal role of cysteines 90 and 102 as exclusive anchoring sites for gold atoms. In the case of C90A a very small peak is visible at +1083 Da (Figure 3A and B).
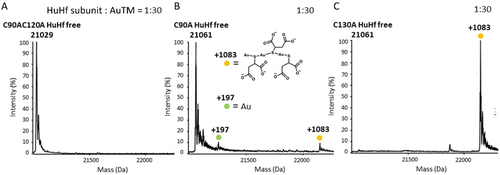
AuTM binding to different HuHf variants. Deconvoluted ESI MS spectra of AuTM bioconjugate with A) C90AC102A HuHf mutant; B) C90A HuHf mutant; C) C130A HuHf mutant. The AuTM : nanocage ratio were: 720 : 1 that correspond to the protein subunit:AuTM ratio of 1 : 30, as indicated in the corresponding panels. The +1083 Da fragment corresponds to the formula Au3TM3Na2; the structural formula of the Au3TM3 species is reported in the first panel.
Conversely, the reaction with the C130A mutant led to complete conversion of the native protein into a metalated species (again with ICP indicating an average of ~2.9 gold atoms per HuHf subunit). Notably, for the latter variant, we detected the presence of a single ESI MS peak for the resulting adduct with a mass of 22146 Da, corresponding to full protein metalation, involving a fragment with a mass of 1083 Da per monomer (Figure 3C). Through spectral simulation, the metallic fragment was found to correspond to the Au3TM3Na2 moiety with a theoretical mass of 1083.8518 Da. This fragment thus appears to contain an additional thiomalate ligand compared to the 935 Da fragment detected in the interaction of AuTM with the wild type apoferritin. This might be due to a partial destabilization of the subunit structure of the C130A variant under the ESI conditions, causing Cys90 to move away from Cys102, and then favouring the binding of an additional TM moiety in the place of a protein Cys to one of the two terminal gold(I) ions.
In order to further characterize the HuHf@AuTM system at the molecular level, the samples of two bioconjugates, i.e., the adduct of AuTM with the wild type protein (HuHf@AuTM) and the corresponding adduct with C130A HuHf, were trypsinized according to a standard procedure27 and the tryptic fragments analyzed by ESI MS in comparison with apo wild type HuHf.
All the expected tryptic fragments (T1-T20) were detected for apo- HuHf and its bioconjugates, with the exception of fragments T3, T4 and T18 (Figure 4). Both Cys90 and Cys102 belong to fragment T12. On trypsinization of either WT or C130A HuHf@AuTM, we detected a peak corresponding to fragment T12 with a mass increase of 1083 Da. This corresponds to the peak observed for the intact C130A adduct (Figure 3C); presumably, the loss of the three-dimensional structure that likely occurs in the tryptic fragment vs the intact subunit causes an increase in the distance between Cys90 and Cys102 permitting the binding of the Au3TM3Na2 moiety. Notably, no metalation was observed on the tryptic fragment T15, which contains residue 130. These observations suggest that metalation occurs exclusively at the level of the portion of the subunits containing the cysteine residues 90 and 102, i.e., the tryptic fragment T12.
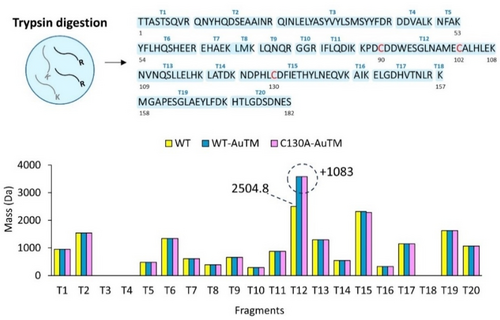
Identification of Au-binding peptides. Upper panel: representation of the tryptic fragments of HuHf (T1-T20); lower panel: results from the ESI-MS analysis of the tryptic fragments of apo WT HuHf (yellow) and of the AuTM bioconjugates with WT HuHf and C130A HuHf (blue and magenta, respectively). The theoretical and experimental masses for each tryptic fragment are provided in Tables S1–S3.
The combined information arising from the ESI MS and ICP measurements performed on the wild type and mutant HuHf@AuTM conjugates as well as from the trypsinization experiments allowed us to develop a computational model of the 3D structure of HuHf@AuTM (Figure 5). Gaussian calculations28 for Au3TM2(CH3S)2 and Au3TM3(CH3S) yielded structural parameters (bond lengths, S−Au−S angles) that closely matched those observed in the X-ray structure,3 although we did not compute a polymeric structure, but only a fragment. Then, the CH3S moieties of Au3TM2(CH3S)2 were replaced by Cys90 and Cys102 to model the species observed for WT HuHf@AuTM.
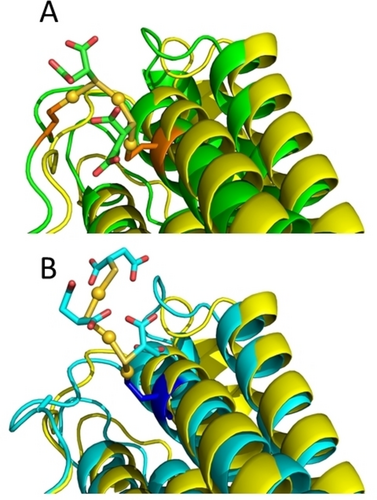
Comparison of the 3D structure of a subunit of ferritin in the absence (yellow) or in the presence of bound Au3TM2 (green, panel A) or Au3TM3 (cyan, panel B). Gold atoms are shown as yellow spheres; the TM ligands are shown as sticks with the same color of the structure backbone. The Cys residues coordinating the Au ions are shown in orange (panel A) or blue (panel B). The Au coordination bonds are shown as yellow sticks. Only a small rearrangement of the flexible loop region harboring Cys90 is needed to accommodate Au3TM2, the largest structural impact being on the bend of the helix containing Cys102 (panel A). The cyan structure in panel B refers to C90A HuHf.
Similarly, the CH3S moiety of Au3TM3(CH3S) was removed to establish a covalent bond with Cys102 to model the minor species observed in the C90A HuHf@AuTM adduct. MD simulations were analyzed in terms of the overall structural stability of the adducts, measured by the displacement of the backbone coordinates of each subunit as a function of the simulation time, as well as the fluctuations of the inter-subunit contacts, which define the quaternary structure of the nanocage.
The imposition of coordination bonds does not alter the tertiary and quaternary structure of the cage. Interestingly, the dynamics of the C90A HuHf adduct as measured by the mean displacement of the Au atoms over time with respect to their average position is comparable to that of HuHf@AuTM despite the latter having one more coordination bond with the protein.
In our structural model (Figure 5) of the adduct a fragment of the type Au3TM2 is associated with the protein through the formation of coordinative Au−S bonds to Cys90 and Cys102. When one of the two Cys is removed (e.g., in the C90A variant) or when the subunit fold is destabilized, thus affecting the distance between C90 and C102 (e.g., in the T12 fragment resulting from trypsinization), we observed an Au3TM3 fragment with a free end and a single Cys-anchor, instead of the Au3TM2 fragment with two-Cys anchoring points on the subunit surface. The lack of metalation on Cys130 is consistent with its buried location inside the 3-fold channels (Figure 1E). These channels facilitate the selective diffusion of small cationic species,29, 30 but are not suitable to host large anionic metal fragments like AuTM.
Antiproliferative and Metabolic Effects of the Adduct vs the Free Drug
The actions of AuTM and of HuHf@AuTM were comparatively investigated in a cellular model of human ovarian cancer, i.e., the A2780 cell line, by the MTT assay. The representative dose-response profiles after 72 h of treatment are shown in Figure S3 together with the corresponding IC50 values. These results point out that conjugation to HuHf produces a huge increase in the action of AuTM toward A2780 cancer cells, amounting to a factor of ~110X (0.43 vs 47 μM), calculated on a molar basis. These IC50 concentrations can halve cell growth, although all cells in the samples result alive when tested via the Trypan Blue assay (Figure S4). To exclude any possible interference due to gold(I) binding to the free Cys34 of human serum albumin (HSA), which is present in the growth media at a concentration ∼60 μM, we investigated by ESI MS the interaction of AuTM-derived gold species with HSA (Figure S5A). Analogously to the case of AF,10 HSA with its single Cys does not compete with HuHf; additionally, no binding to HSA is observed even when incubating HSA with free AuTM up to a HSA : AuTM ratio of 1 : 5, which is larger than that used for cell treatment (Figure S5B).
To support this hypothesis and demonstrate that the association with ferritin may make AuTM more selective for cancer cells than for healthy cells, we have comparatively measured the level of transferrin receptor expression in A2780 cells and in Human Embryonic Kidney (HEK) cells, the latter taken as a model of non-cancerous cell lines. Consistently with literature data,18, 31, 32 TfR1 was found to be expressed in significantly higher levels in A2780 cells (Figure 6A). Using ferritin labeled with the Alexa Fluor 488 dye, we could observe the more efficient uptake of the nanocage by A2780 cells with respect to HEK cells (Figure 6B). In line with these results, the IC50 at 72 h for HEK cells treated with HuHf@AuTM is >4 μM (on a ferritin molar basis), i.e., at least 10 times larger than the corresponding value for the A2780 cells.
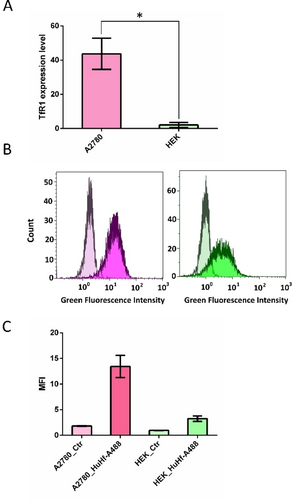
TfR1-mediated uptake of HuHf. A) Bar plots (mean value±SD) of the TfR1 expression level in A2780 cells and HEK 293 cells determined by Western Blot analysis (t-test, n=3). B–C) Flowcytometry analysis of A2780 and HEK 293 cells treated with HuHf-Alexa 488 for 1 h at 37 °C. B) Distribution of the fluorescence intensity C) Bar plots (mean value±SD, n=2) of the median fluorescence intensity (MFI) derived from panel B). Controls/treatments for A2780 and HEK 293 are shown in light/dark magenta and light/dark green, respectively.
Figure 7 shows the MTT time course (24, 48 and 72 h) performed upon treating A2780 cells with AuTM and HuHf@AuTM with the respective 72 h-IC50 values, whereas Figure 7B compares the cellular gold uptake measured through ICP-OES determinations, at the same times of treatment and equal compound concentrations as for the MTT time course. Gold concentrations were determined on mineralized cellular pellets derived from viable cells (see Supporting Information for the detailed procedure). It is evident that the treatment with the HuHf@AuTM adduct resulted in a much higher cellular uptake of gold compared to the free drug (by a factor ~7.5 at 24 h, which decreased down to ∼3.0 at 48 h). The more efficient gold uptake might explain the differences in IC50. These data strongly suggest that ferritin acts as an excellent nanocarrier for AuTM, greatly enhancing the effect of the free drug.
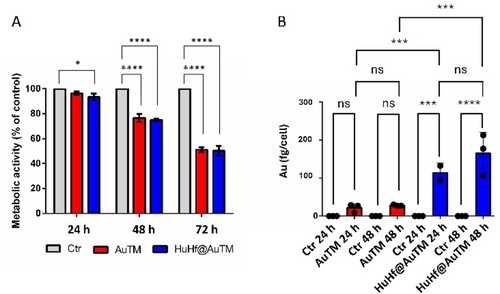
Time course analysis of the metabolic activity and gold uptake in A2780 cells. A) Metabolic MTT assay upon treatment with AuTM and HuHf@AuTM for 24, 48, and 72 h, with the respective 72 h-IC50 concentrations. The histograms represent the mean±SD values of the percentage of the metabolic activity of treated cells relative to untreated controls. * : p-value<0.05; **** : p-value<0.0001(Tukey's multiple comparison test, n=6). B) Amount of intracellular gold measured by ICP-OES in untreated cells and following treatment with AuTM and HuHf@AuTM for 24 and 48 h, with the respective 72 h-IC50 concentrations; *** : p-value<0.001; **** : p-value<0.0001 (Tukey's multiple comparison test, n=3). Color coding: grey=untreated (Ctr); red= treated with AuTM; blue=treated with HuHf@AuTM.
Afterward, to gain deeper insight into how the treatment with free AuTM and HuHf@AuTM affects cellular processes, untargeted 1H NMR metabolomic analysis was performed both on cell lysates and growth media of A2780 cells treated with AuTM or with HuHf@AuTM at two different time points, 24 h and 48 h, using the respective IC50 concentrations at 72 h (equal toxicity). Representative 1H NMR spectra of cell lysates and growth media of untreated (control) cells and treated A2780 cells are provided in Figure S6 and Figure S7. The assigned metabolites are listed in Table S4. The pairwise comparisons between A2780 control cells and AuTM- or HuHf@AuTM-treated cells revealed significant alterations in response to these treatments, both in the media and in the lysates (Figure S8). The time dependence of the significant changes is depicted by the box plots of Figures 8 and 9, assembling changes as a function of glycolytic and TCA pathways, respectively.
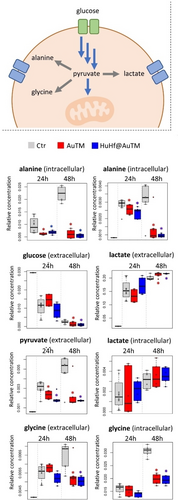
Changes in the levels of metabolites related to glycolysis. The box plots show the variations in the NMR-determined relative concentrations of the metabolites in cell lysates (intracellular) and growth media (extracellular), after 24 and 48 h of treatment with free AuTM and HuHf@AuTM in comparison to untreated (Ctr) cells. The top panel summarizes the relationship among these metabolites. Red asterisks: p-value<0.05 for the comparison Ctr vs. AuTM-treated cells; Blue asterisks: p-value <0.05 for the comparison Ctr vs. HuHf@AuTM-treated cells (pairwise Wilcoxon–Mann–Whitney test, n=6). Color coding of the box plots: grey=untreated (Ctr); red= treated with AuTM; blue=treated with HuHf@AuTM.

Changes in metabolites and proteins related to the TCA cycle. The bar plots of SDHA and CS expression levels are reported together with the boxplots of the intra- and extra- cellular concentration levels of succinate, fumarate and citrate after 24 h and 48 h of AuTM and HuHf@AuTM treatments. The protein levels are reported in % with respect to the control cells. The central panel summarizes the relationship among these metabolites and enzymes. Red asterisks: p-value<0.05 for the comparison Ctr vs AuTM; Blue asterisks: p-value<0.05 for the comparison Ctr vs HuHf@AuTM (pairwise Wilcoxon–Mann–Whitney test, n=6). Color coding of the box plots: grey=untreated (Ctr); red= treated with AuTM; blue=treated with HuHf@AuTM.
The most relevant changes induced by AuTM and HuHf@AuTM treatment in A2780 cancer cells based on our NMR metabolomics data are summarised below:
- –
The changes induced by AuTM and HuHf@AuTM are qualitatively very similar.
- –
Most of the changes detected at 48 h with free AuTM are already visible at 24 h with HuHf@AuTM treatment, consistent with the faster metal uptake already described.
- –
A significant increase in intracellular GSH levels is observed, which is one of the main similarities with the metabolomic effects induced by AF10, 33 (Figure S9), although the absolute concentration of GSH in AuTM-treated cells is much smaller than for AF-treated cells.
- –
Increased uptake of glucose together with increased release of lactate at 48 hours suggests an increase in the glycolytic activity (Figure 8). This is accompanied by a significant reduction in pyruvate release and lower intracellular concentrations of Ala and Gly. In fact, pyruvate, besides being reduced to lactate, can be transaminated to produce alanine or redirected to glycine biosynthesis.
- –
The intracellular increase of succinate and the decrease of fumarate, together with an increased release of succinate in the media during cell growth, are indicative of an AuTM-dependent dysregulation of the TCA cycle starting from 24 h. At 48 h of treatment, we also observed lower intracellular levels of citrate and, accordingly, a reduced extracellular release of this metabolite (Figure 9).
To validate the conclusions drawn from the NMR metabolomics analysis, we carried out additional biochemical tests. In a first experiment we evaluated whether AuTM treatment may affect oxygen consumption as previously observed for AF and the related compounds gold(I) monocarbene and gold(I) dicarbene.34 The results (Figure S10) indicated that AuTM did not affect oxygen consumption at all. Then, to confirm the alterations observed in the TCA cycle metabolites, succinate dehydrogenase (SDHA) and citrate synthase (CS) protein levels were measured in treated cells versus controls (Figure 9). A significant decrease in SDHA is observed for both treatments at both time points; this behaviour explains the observed increase in succinate (substrate) and decrease in fumarate (product) levels. A progressive decrease of CS is observed over time for both treatments, which is consistent with a decrease in citrate; Acetyl-CoA is below the detection limit of 1H NMR.
Therefore, by integrating untargeted NMR and targeted biochemical tests, we obtained a fully consistent demonstration that AuTM and HuHf@AuTM induce substantially similar alterations in the metabolome. In turn, the latter differ profoundly from those previously reported for AF33 and its ferritin adduct10 as well as for the gold(I) monocarbene and gold(I) dicarbene compounds.34
Finally, we tested the activity of AuTM and HuHf@TM in a cellular model of A2780 cells resistant to cisplatin (named A2780cis, Figure S11A and B). Similarly to what observed for cisplatin sensitive A2780 cells, HuHf@AuTM has a much stronger effect than free AuTM, of the order of 60X, calculated on a molar basis. Both AuTM and HuHf@AuTM appear to overcome cisplatin resistance. Consistently, the analysis based on metabolomics shows that AuTM and its bioconjugate affect largely different pathways (Figure S11C) than cisplatin.
Conclusion
AuTM is an established gold-based drug with a long history in the clinical treatment of rheumatoid arthritis.1 In addition, AuTM has shown encouraging anti-cancer properties both in vitro and in vivo and has progressed to clinical trials,8 although it is less prone to enter cancer cells than other gold-based compounds like AF.9, 35 The idea behind this study is to exploit ferritin as a nanocarrier for AuTM to obtain a better drug uptake thanks to the internalization of HuHf mediated by the TfR1 receptor. The latter is expressed at high levels in several tumor cell lines.
In this work, we have shown that a well-defined adduct between AuTM and ferritin (HuHf@AuTM) can be prepared according to a standardized protocol. The adduct has been characterized in detail, at the molecular level, by ESI MS and ICP measurements. In particular, the adduct contains a peculiar Au3 nanocluster tightly associated with cysteines 90 and 102. This interpretation is well supported by computational modeling and MD simulations. The association with ferritin strongly enhances the cytotoxicity of the free drug by a factor of ~110, when calculated on a molar basis, which can be interpreted in terms of a far more efficient drug uptake when receptor mediated. However, based on NMR metabolomics results, the overall effects of the adduct on cancer cell metabolism strictly resemble those of the free drug, suggesting the occurrence of a common mode of action. The effects at the level of the metabolome are interpreted as a major alteration of the TCA cycle, while the mitochondrial function is not significantly affected. The described adduct represents an exemplary case of a ferritin-drug conjugate with enhanced pharmacological properties and increased selectivity for cancer cells.
Supporting Information
The authors have cited additional references within the Supporting Information.28, 30, 33, 36-44
Acknowledgments
We gratefully acknowledge funding by AIRC, IG 2021 ID 26169 “Multi-Omics approach to establish the molecular mechanisms of Anticancer Gold Compounds in the Systems Biology Era”. P.T., A.R., A.G., G.D.P., V.G., L.C. and S.Z. acknowledge the support and the use of resources of Instruct-ERIC, a Landmark ESFRI project, and specifically the CERM/CIRMMP Italy Centre, and the project “Potentiating the Italian Capacity for Structural Biology Services in Instruct-ERIC, Acronym “ITACA.SB” (Project no. IR0000009) within the call MUR 3264/2021 PNRR M4/C2/L3.1.1, funded by the European Union – NextGenerationEU. This work was co-funded by the European Union—Next GenerationEU, in the context of the National Recovery and Resilience Plan (PNRR), Investment 1.5 Ecosystems of Innovation, project Tuscany Health Ecosystem (THE), Spoke 6. The activity is also related to the research contract co-funded by the European Union—PON Research and Innovation 2014–2020 in accordance with Article 24, paragraph 3a), of Law No. 240 of December 30, 2010, as amended, and Ministerial Decree No. 1062 of August 10, 2021. This work has received funding from the Italian Ministry of Education and Research (MUR), through Department di Eccellenza 2023–2027 (DICUS 2.0) to the Department of Chemistry “Ugo Schiff” of the University of Florence. S.Z. contributed to this work before the start of his PhD. Dr. Valentina Vitali is gratefully acknowledged for assistance in performing the trypsinization experiments. Open Access publishing facilitated by Università degli Studi di Firenze, as part of the Wiley - CRUI-CARE agreement.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.