Development of a Dual-Factor Activatable Covalent Targeted Photoacoustic Imaging Probe for Tumor Imaging
Dr. Jiho Song
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
These authors contributed equally to this work.
Search for more papers by this authorTianqu Zhai
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Heung Sik Hahm
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorDr. Yuancheng Li
Department of Radiology and Imaging Science, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorProf. Dr. Hui Mao
Department of Radiology and Imaging Science, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorProf. Dr. Xueding Wang
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
Search for more papers by this authorCorresponding Author
Dr. Janggun Jo
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jae Won Chang
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
Department of Hematology and Medical Oncology, Emory University, Atlanta, Georgia, 30322 United States
Winship Cancer Institute, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorDr. Jiho Song
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
These authors contributed equally to this work.
Search for more papers by this authorTianqu Zhai
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Heung Sik Hahm
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorDr. Yuancheng Li
Department of Radiology and Imaging Science, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorProf. Dr. Hui Mao
Department of Radiology and Imaging Science, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorProf. Dr. Xueding Wang
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
Search for more papers by this authorCorresponding Author
Dr. Janggun Jo
Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jae Won Chang
Department of Pharmacology and Chemical Biology, Emory University, Atlanta, Georgia, 30322 United States
Department of Hematology and Medical Oncology, Emory University, Atlanta, Georgia, 30322 United States
Winship Cancer Institute, Emory University, Atlanta, Georgia, 30322 United States
Search for more papers by this authorGraphical Abstract
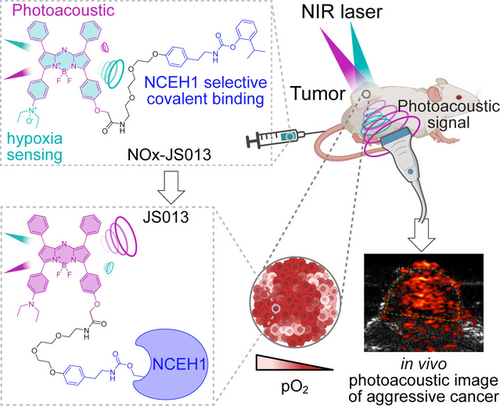
We developed an NCEH1-targeted covalent photoacoustic imaging probe activated under hypoxia. This probe potently and selectively labels NCEH1, an aggressive tumor associated serine hydrolase, in cells and is also activated under hypoxic conditions. Through covalent labeling and hypoxia activation, this probe offers selective and high-quality in vivo tumor imaging of aggressive prostate cancer.
Abstract
Photoacoustic imaging (PAI) is an emerging modality in biomedical imaging with superior imaging depth and specificity. However, PAI still has significant limitations, such as the background noise from endogenous chromophores. To overcome these limitations, we developed a covalent activity-based PAI probe, NOx-JS013, targeting NCEH1. NCEH1, a highly expressed and activated serine hydrolase in aggressive cancers, has the potential to be employed for the diagnosis of cancers. We show that NOx-JS013 labels active NCEH1 in live cells with high selectivity relative to other serine hydrolases. NOx-JS013 also presents its efficacy as a hypoxia-responsive imaging probe in live cells. Finally, NOx-JS013 successfully visualizes aggressive prostate cancer tumors in mouse models of PC3, while being negligibly detected in tumors of non-aggressive LNCaP mouse models. These findings show that NOx-JS013 has the potential to be used to develop precision PAI reagents for detecting metastatic progression in various cancers.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202410645-sup-0001-misc_information.pdf6.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Hernot, L. van Manen, P. Debie, J. S. D. Mieog, A. L. Vahrmeijer, Lancet Oncol. 2019, 20, e354–e367.
- 2J. V. Frangioni, J. Clin. Oncol. 2008, 26, 4012–4021.
- 3A. S. Thakor, S. S. Gambhir, Ca-Cancer J. Clin. 2013, 63, 395–418.
- 4S. Mallidi, G. P. Luke, S. Emelianov, Trends Biotechnol. 2011, 29, 213–221.
- 5K. Paydary, S. M. Seraj, M. Z. Zadeh, S. Emamzadehfard, S. P. Shamchi, S. Gholami, T. J. Werner, A. Alavi, Mol. Imaging Biol. 2019, 21, 1–10.
- 6S. Venneti, M. P. Dunphy, H. W. Zhang, K. L. Pitter, P. Zanzonico, C. Campos, S. D. Carlin, G. La Rocca, S. Lyashchenko, K. Ploessl, D. Rohle, A. M. Omuro, J. R. Cross, C. W. Brennan, W. A. Weber, E. C. Holland, I. K. Mellinghoff, H. F. Kung, J. S. Lewis, C. B. Thompson, Sci. Transl. Med. 2015, 7, 274ra17.
- 7L. Zhu, K. Ploessl, R. Zhou, D. Mankoff, H. F. Kung, J. Nucl. Med. 2017, 58, 533–537.
- 8Y. Yang, H. Hong, Y. Zhang, W. Cai, Cancer Growth Metastasis 2009, 2, 13–27.
- 9M. Vizovisek, R. Vidmar, M. Drag, M. Fonovic, G. S. Salvesen, B. Turk, Trends Biochem. Sci. 2018, 43, 829–844.
- 10G. A. M. S. van Dongen, A. J. Poot, D. J. Vugts, Tumor Biol. 2012, 33, 607–615.
- 11Z. Szabo, E. Mena, S. P. Rowe, D. Plyku, R. Nidal, M. A. Eisenberger, E. S. Antonarakis, H. Fan, R. F. Dannals, Y. Chen, R. C. Mease, M. Vranesic, A. Bhatnagar, G. Sgouros, S. Y. Cho, M. G. Pomper, Mol. Imaging Biol. 2015, 17, 565–574.
- 12S. Manohar, S. S. Gambhir, Photoacoustics 2020, 19, 100196.
- 13Y. Liu, L. Teng, B. Yin, H. Meng, X. Yin, S. Huan, G. Song, X. B. Zhang, Chem. Rev. 2022, 122, 6850–6918.
- 14S. Wang, Z. Sheng, Z. Yang, D. Hu, X. Long, G. Feng, Y. Liu, Z. Yuan, J. Zhang, H. Zheng, X. Zhang, Angew. Chem. Int. Ed. 2019, 58, 12415–12419.
- 15P. Cheng, W. Chen, S. Li, S. He, Q. Miao, K. Pu, Adv. Mater. 2020, 32, 1908530.
- 16Z. X. Zhao, C. B. Swartchick, J. Chan, Chem. Soc. Rev. 2022, 51, 829–868.
- 17C. Qiu, Y. Bai, T. Yin, X. Miao, R. Gao, H. Zhou, J. Ren, L. Song, C. Liu, H. Zheng, R. Zheng, BMC Cancer 2020, 20, 419.
- 18S. Zhang, H. Chen, L. Wang, X. Qin, B. P. Jiang, S. C. Ji, X. C. Shen, H. Liang, Angew. Chem. Int. Ed. 2022, 61, e202107076.
- 19S. H. Gardner, C. J. Brady, C. Keeton, A. K. Yadav, S. C. Mallojjala, M. Y. Lucero, S. Su, Z. Yu, J. S. Hirschi, L. M. Mirica, J. Chan, Angew. Chem. Int. Ed. 2021, 60, 18860–18866.
- 20L. V. Wang, S. Hu, Science 2012, 335, 1458–1462.
- 21A. K. East, M. C. Lee, C. Jiang, Q. Sikander, J. F. R. Chan, J. Am. Chem. Soc. 2023, 145, 7313–7322.
- 22J. D. Zheng, Q. Zeng, R. J. Zhang, D. Xing, T. Zhang, J. Am. Chem. Soc. 2019, 141, 19226–19230.
- 23L. L. Teng, G. S. Song, Y. C. Liu, X. Y. Han, Z. Li, Y. J. Wang, S. Huan, X. B. Zhang, W. H. Tan, J. Am. Chem. Soc. 2019, 141, 13572–13581.
- 24K. P. Chiang, S. Niessen, A. Saghatelian, B. F. Cravatt, Chem. Biol. 2006, 13, 1041–1050.
- 25D. K. Nomura, J. Z. Long, S. Niessen, H. S. Hoover, S. W. Ng, B. F. Cravatt, Cell 2010, 140, 49–61.
- 26N. Jessani, Y. S. Liu, M. Humphrey, B. F. Cravatt, Proc. Natl. Acad. Sci. USA 2002, 99, 10335–10340.
- 27B. F. Cravatt, A. T. Wright, J. W. Kozarich, Annu. Rev. Biochem. 2008, 77, 383–414.
- 28J. W. Chang, D. K. Nomura, B. F. Cravatt, Chem. Biol. 2011, 18, 476–484.
- 29J. W. Chang, M. J. Niphakis, K. M. Lum, A. B. Cognetta 3rd, C. Wang, M. L. Matthews, S. Niessen, M. W. Buczynski, L. H. Parsons, B. F. Cravatt, Chem. Biol. 2012, 19, 579–588.
- 30J. W. Chang, A. B. Cognetta 3rd, M. J. Niphakis, B. F. Cravatt, ACS Chem. Biol. 2013, 8, 1590–1599.
- 31A. B. Cognetta 3rd, M. J. Niphakis, H. C. Lee, M. L. Martini, J. J. Hulce, B. F. Cravatt, Chem. Biol. 2015, 22, 928–937.
- 32A. F. Kornahrens, A. B. Cognetta 3rd, D. M. Brody, M. L. Matthews, B. F. Cravatt, D. L. Boger, J. Am. Chem. Soc. 2017, 139, 7052–7061.
- 33J. Fan, S. Guo, S. Wang, Y. Kang, Q. Yao, J. Wang, X. Gao, H. Wang, J. Du, X. Peng, Chem. Commun. 2017, 53, 4857–4860.
- 34J. W. Chang, R. E. Moellering, B. F. Cravatt, Angew. Chem. Int. Ed. 2012, 51, 966–970.
- 35J. W. Chang, M. Bhuiyan, H. M. Tsai, H. J. Zhang, G. Li, S. Fathi, D. C. McCutcheon, L. Leoni, R. Freifelder, C. T. Chen, R. E. Moellering, Angew. Chem. Int. Ed. 2020, 59, 15161–15165.
- 36K. Ruan, G. Song, G. Ouyang, J. Cell. Biochem. 2009, 107, 1053–1062.
- 37C. Wigerup, S. Påhlman, D. Bexell, Pharmacol. Ther. 2016, 164, 152–169.
- 38H. J. Knox, J. Hedhli, T. W. Kim, K. Khalili, L. W. Dobrucki, J. Chan, Nat. Commun. 2017, 8, 1794.
- 39C. R. Nishida, P. R. O. de Montellano, J. Med. Chem. 2008, 51, 5118–5120.
- 40D. Kang, S. T. Cheung, A. Wong-Rolle, J. Kim, ACS Cent. Sci. 2021, 7, 631–640.
- 41A. Kamkaew, K. Burgess, Chem. Commun. 2015, 51, 10664–10667.
- 42D. Frath, P. Didier, Y. Mély, J. Massue, G. Ulrich, ChemPhotoChem 2017, 1, 109–112.
- 43J. Murtagh, D. O. Frimannsson, D. F. O'Shea, Org. Lett. 2009, 11, 5386–5389.
- 44M. E. C. Biffin, J. Miller, D. B. Paul, Tetrahedron Lett. 1969, 1015–1018.
- 45M. E. C. Biffin, G. Bockstei, J. Miller, D. B. Paul, Aust. J. Chem. 1974, 27, 789–799.
- 46R. B. P. Elmes, Chem. Commun. 2016, 52, 8935–8956.
- 47L. H. Patterson, Cancer Metastasis Rev. 1993, 12, 119–134.
- 48D. L. Kirkpatrick, H. L. Schroeder, K. J. Chandler, Anti-Cancer Drugs 1994, 5, 467–472.
- 49T. Hirayama, H. Tsuboi, M. Niwa, A. Miki, S. Kadota, Y. Ikeshita, K. Okuda, H. Nagasawa, Chem. Sci. 2017, 8, 4858–4866.
- 50M. Niwa, T. Hirayama, K. Okuda, H. Nagasawa, Org. Biomol. Chem. 2014, 12, 6590–6597.
- 51D. G. Tew, Biochemistry 1993, 32, 10209–10215.
- 52P. Phukhum, J. Phetcharaburanin, K. Chaleekarn, Y. Kittirat, T. Kulthawatsiri, N. Namwat, W. Loilome, N. Khuntikeo, A. Titapun, A. Wangwiwatsin, T. Khampitak, M. Suksawat, P. Klanrit, Sci. Rep. 2023, 13, 3072.
- 53J. Jo, C. H. Lee, R. Kopelman, X. D. Wang, Nat. Commun. 2017, 8, 471.
- 54L. F. Portugal, J. J. Judice, L. N. Vicente, Math. Comput. 1994, 63, 625–643.
- 55I. Elfaki, R. Mir, F. M. Almutairi, F. M. A. Duhier, Asian Pacific J. Cancer Prev. 2018, 19, 2057–2070.
- 56D. J. Lim, X. L. Liu, D. M. Sutkowski, E. J. Braun, C. Lee, J. M. Kozlowski, Prostate 1993, 22, 109–118.