Living Hybrid Exciton Materials: Enhanced Fluorescence and Chiroptical Properties in Living Supramolecular Polymers with Strong Frenkel/Charge-Transfer Exciton Coupling
Graphical Abstract
Asymmetrically L/D-glutamate-derived PDIs having linear alkyl chains exhibit living supramolecular polymerization in thermodynamically stable chiral molecular orientation, which shows the coupling of Frenkel and charge transfer exciton states, resulting in significantly enhanced fluorescence quantum yield, CD and CPL properties. Meanwhile, PDIs with branched chains only show common H-aggregates. Thus, molecular control on living hybrid exciton nanomaterials is achieved.
Abstract
A family of chiral perylene diimides (PDIs) was newly developed as excellent circularly polarized luminescence (CPL) materials. They are asymmetrically derivatized with a double-alkyl-chained L- or D-glutamate unit and a linear or branched alkyl chain. When water is added to the tetrahydrofuran (THF) solution of glutamate-PDI-linear-alkyl chain compounds, kinetically formed H-aggregates are formed in globular nanoparticles (NPs). These NPs undergo spontaneous transformation into thermodynamically stable nanotubes via helical nanostructures, which showed structured broad spectra originating from the strong coupling of delocalized Frenkel excitations (FE) and charge transfer excitations (CTE). Significant enhancement of circular dichroism (CD), fluorescence quantum yield, and circularly polarized luminescence (CPL) with luminescence dissymmetry factor (glum) are observed during the transformation of NPs to the FE/CTE-coupled helical and tubular structures. This transformation process is significantly accelerated by applying physical stimuli, i.e., ultrasonication or adding helical aggregates as seed crystals, a feature unique to living supramolecular polymerization. Meanwhile, the branched chain-containing PDIs only form H-aggregates and did not show FE/CTE hybrid exciton states with living supramolecular polymerization properties. This study unveils that suitably designed chiral PDI derivatives show FE/CTE coupling accompanied by high fluorescence quantum yields, enhanced chiroptical properties, and supramolecular living polymerization characteristics.
Introduction
Chiroptical materials are an important family of functional materials with various applications.1 Essential characteristics of chiroptical material are circular dichroism (CD) and circularly polarized luminescence (CPL),2 where CD is the differential absorption of left- and right-handed circularly polarized light by chiral molecule, and it has been widely utilized to probe the ground state chirality.3 On the other hand, CPL indicates the different emission intensities of circularly polarized light from chiral luminescent molecules, providing information on the chirality in the excited state. The application of CPL has recently gained increasing interest in modern technologies such as optical devices for 3D displays,4 information storage, encryption,5 and asymmetric organic synthesis.6
One of the unsolved challenges in chiroptical materials is achieving high dissymmetry factors (absorption dissymmetry factors gCD and luminescence dissymmetry factor glum) and high fluorescence quantum yield.7 Molecular self-assembly8 has been one of the most efficient approaches to enhance dissymmetry factors.8a, 8b, 9 Meanwhile, fluorescent quantum yields (Φfl) of self-assemblies generally drop compared to those of the monomeric chromophores in solution, and a trade-off exists between glum and Φfl.7, 8b, 10 The ultimate solution to this problem is a methodology to suppress the formation of nonradiative energy traps that reduce fluorescence quantum yields. It will require the proper selection of chromophores and the ability to control kinetic and thermodynamic association processes, as demonstrated for living supramolecular polymerization systems.11 An essential molecular technology underlying the realization of these challenges is controlling the excitonic states’ nature based on precise control over the intermolecular distance and orientation of chiral chromophores. However, supramolecular living polymerization of chiral chiroptical molecules that show high glum and Φfl remains a significant challenge in this field.7b, 12
In this study, we report on asymmetrically designed, chiral perylene diimides (PDIs) that show living supramolecular polymerization with hybrid Frenkel/charge transfer exciton (FE/CTE) properties. The living supramolecular polymerization was accompanied by enhancing chiroptical properties (gCD, glum) and fluorescence quantum yields(Φfl). We employed perylene diimides (PDIs) because they show high thermal and photochemical stabilities, intense absorptions in the visible region, high intrinsic fluorescence quantum yield in solution, and the formation of π-stacked aggregates.13 The PDI assemblies show excitonic interactions reflected in the spectroscopic properties. Würthner and co-workers reported living supramolecular polymerization of PDIs with symmetrically introduced solvophilic moieties.14 Kawai and others noted that chiral PDI dimers exhibit enhancement of CPL upon aggregation. However, in these studies, the aggregation occurred at the cost of significantly reducing fluorescence quantum yield (Φfl) (see Supporting Information Figure S1 and Table S1).9g, 15 Sanchez and co-workers reported seeded supramolecular polymerization of N-annulated PDIs,16 but no amplification of dissymmetry was observed.16b Despite the progress of supramolecular polymers in recent years, the rational control of fluorescence quantum yield and chiroptical properties in supramolecular living polymerization systems is still in its infancy.12e, 17
We developed a new family of chiral PDIs by introducing double-chained chiral glutamate and achiral units at both ends of the chromophore (Scheme 1). A glutamate group with flexible and solvophilic ether-linked alkyl chains18 was attached to one of the imide positions, and the other side was modified with linear or branched achiral alkyl chains. Living supramolecular polymerization was successfully achieved for the chiral glutamate-PDI compounds having n-alkyl chains. They showed a dynamic transformation from kinetically formed nanoparticles (H-aggregates, Hagg) to thermodynamically stable, regular helical nanofibers which showed a broad, structured spectrum caused by the coupling of delocalized Frenkel excitations (FE) and charge transfer excitation (CTE) states (JCT-state). Interestingly, this transformation was accompanied by a ca. 10-fold enhancements of fluorescence quantum yield (1.2 % to 13 %) and |gCD| value, which was also accelerated by the physical stimulus of ultrasonication or by adding a nanocrystalline seed of shortened nanofibers, characteristic features of living supramolecular polymerization. In stark contrast, PDIs with branched chains stayed as H-aggregated nanoparticles without structural transformation despite ultrasonic irradiation and the addition of seeds. Thus, living supramolecular polymerization of FE/CTE hybrid exciton materials with enhanced chiroptical properties has been achieved based on molecular design.
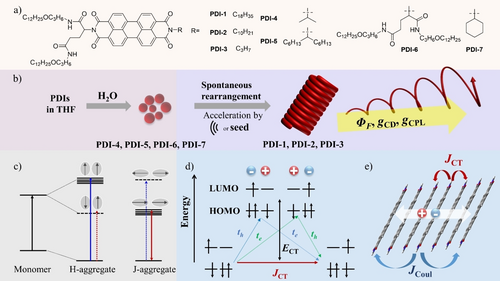
(a) Molecular structures of chiral PDI compounds, (b) Schematic self-assembly behavior of PDIs in THF/H2O, (c) Classical Kasha exciton coupling model for H- and J-aggregates based on point-dipole approximation and Coulombic coupling, (d) Energy level diagram depicting charge transfer interactions between neighboring chromophores in Spano model, showing the interference between the long-range Coulombic interactions (JCoul) and short-range charge-transfer (CT)-mediated interactions (JCT). JCT results from the sum of the blue and green virtual two-step pathways. (e) A schematic illustration of the Frenkel/CT mixed exciton-coupled hybrid exciton state in tilt PDI chromophores with CT and Coulombic interactions. This CT-mediated assembled state is referred to as the JCT-state in this work.
Results and Discussion
Self-Assembly and the Emergence of Frenkel/Charge Transfer-Coupled Exciton State
The asymmetric PDIs were synthesized in one step from perylene dianhydride and corresponding amine derivatives, which were isolated by column chromatography (Scheme 1, and Experimental Section in Supporting Information). L-PDI-1 dissolved in THF shows sharp 0→0 (522 nm), 0→1 (486 nm), and 0→2 (455 nm) vibronic transitions typical of the monomeric species (Figure 1a and Figure S2), and monomer emission with vibrational structure (Figure 1b). This absorption band is associated with the dipole moment polarized along the long (N−N) axis of the PDI chromophore.19 To promote the self-assembly of L-PDI-1, H2O was added to the THF solution with different volume fractions, and their Vis absorption and photoluminescence spectra (PL) were measured (Figures 1, S2, S3). The color of the solutions changed from yellow (monomer solution in THF) to red upon increasing the fraction of H2O above 50 v/v% (Figure S3a), indicating the formation of aggregates at higher H2O contents. The absorption spectrum of the aggregates shows the disappearance of the sharp 0→0 (522 nm), 0→1 (486 nm), and 0→2 (455 nm) vibronic transitions observed in THF, giving a broad absorption maximum near 500 nm and a shoulder peak around 550 nm (Figure 1a and Figure S2). This absorption showed a considerable hypochromism compared to the absorption spectrum of the monomer. The observed blue shifts and hypochromism in the absorption peak are commonly observed in π-stacked PDI chromophores, which have been assigned to H-aggregates according to Kasha's exciton coupling model.20 The exciton coupling in our system is confirmed by a positive and negative Cotton effect in CD spectra (Figure S4). These data indicate that the PDI cores assume parallel-aligned orientation with a slight twist in the long-axis dipole moments induced by the chiral glutamate units.3 Consistent with the observed aggregate formation shown in the absorption spectra, a broad and red-shifted fluorescence, typical of excimer emission, was newly observed around 691 nm (Figure 1b and Figure S3c).
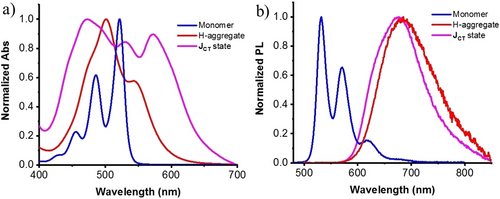
(a) Normalized absorption (a) and PL (b) spectra of L-PDI-1 monomer in THF, Hagg and JCT state in THF/H2O (3 : 7, V/V). Conditions: [c]=2.5×10−5 M.
Depending on the solvent composition, these aggregates were dispersed in the THF/H2O mixtures for several hrs to days (Figure S5a). The dispersions with a water content (VH2O) of 40–80 v/v % gave precipitates after 2 days of preparation, which were facilely redispersed by ultrasonic irradiation using a bath-type ultrasonicator. The incubated samples at a water content of 40 vol % showed a new complex absorption peak at 570 nm, in addition to the absorptions at 488, 525 nm, which are close to 0→1 and 0→0 vibronic transitions of the monomeric PDI chromophore (Figure S5b). The change in the absorption spectrum is accompanied by the appearance of an intense red emission peak at 636 nm in addition to the monomeric emission peaks at 535 and 578 nm (Figure S5c). The aqueous mixture with 40 vol % water appears to be an intermediate state between the 30 vol % water (molecularly dispersed state) and the 50 vol % water (aggregated state) specimens. Upon aging the mixtures at higher water contents of 50–80 vol % (Figure 1a and Figure S5b), two prominent absorption peaks are observed at 471 nm and 570 nm with almost similar intensities, which are located at shorter and longer wavelengths compared to the peak wavelengths of the H-aggregate (500 nm) and the 0–0 absorption of the monomer (522 nm). In addition, significant absorption intensity was observed in the wavelength region between the observed absorption splitting to the higher and lower-energy level exciton bands. These unique absorption spectral changes cannot be represented by classical Kasha's exciton model (Scheme 1c), which is based on the simple Coulombic coupling and point-dipole approximation.21 The observed broad spectra with both the blue-shifted and red-shifted peaks in Figures 1a and S5b (VH2O, 40–80 %) can be explained by considering the strong coupling between long-ranged Frenkel excitons (FE) and short-ranged charge transfer excitons (CTE, Scheme 1d, e).22 In FE, the electron in the excited state and the hole in the ground state reside on the same chromophore, whereas in CTE, the electron and hole reside on different chromophores (Scheme 1d). Such mixing occurs when the energy levels of FEs and CTEs are close, so the diabatic CT states interact strongly via indirect coupling through the diabatic Frenkel exciton.22 The sufficient absorption intensity between the 471 nm and 570 nm peaks indicates an extended CT state in which electrons and holes can be separated by a considerable distance.22 It is reasonable that such FE-CT mixing is promoted in aqueous mixtures with higher water content since the contribution of polar CT states increases with the effective dielectric constants of the medium. Similar broad absorption spectra can be found for self-assembled perylene chromophores.14d, 14g, 23
In CD spectra, monomeric L-PDI-1 in aqueous THF below the water content of 30 vol % showed no CD signals in the S0–S1 transition band (Figure S4). This indicates that the point chirality of the L-glutamide group does not provide a chiral environment to PDI cores. Meanwhile, exciton-coupled CD signals were observed for initially formed H-aggregates at higher water contents (Figure S4), with a |gCD| value of ∼2.2×10−3 at 465 nm (Figure S6), indicating the rotational displacement of the PDI chromophores in the aggregates. Meanwhile, upon aging, more intense CD signals were obtained (Figure 2c, Figure S6, S8) with 10-fold enhancement of the |gCD| value at 570 nm (∼2.1×10−2) (Figure S6). Mirror-imaged CD spectra were observed for L-PDI-1 and D-PDI-1, respectively. We found that the CD spectra of the JCT state show linear dichroism (LD) components (Figures S7, S8). Still, the mirror-symmetry CD curves observed indicate that the contribution of LD is not so significant as to affect the conclusion of the CD spectra (Figure S8). The middle absorption peak around 528 nm also exists in the CD spectra, supporting that the moderately intense absorption component reflects the FE-CTE mixing in the chiral PDI assemblies. The observed changes in the spectral pattern and significantly enhanced CD intensity are consistent with the formation of the mixed FE/CTE state in the incubated samples, which implies a slightly slipped orientation of the perylene chromophores relative to the initial H-aggregates.22 In this paper, the molecular orientation obtained after aging is referred to as a JCT state to account for the contribution of CT excitons.
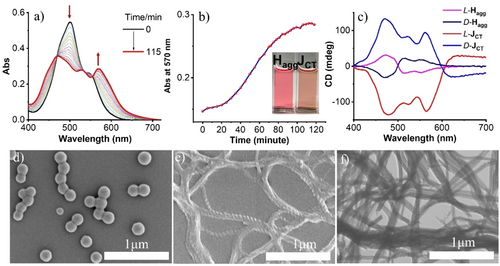
(a) Time-dependent absorption spectral changes of PDI-1. (b) A plot of time-dependent absorbance at 570 nm. Inset is the picture of freshly prepared Hagg and JCT-state after the transformation. (c) CD spectra of freshly prepared Hagg and JCT after incubation for 2 hrs. (d) SEM images of freshly prepared Hagg. (e) JCT-state after the transformation. (f) A TEM image of the aggregates in JCT-state. Conditions: [PDI-1]=2.5×10−5 M. Water content is 70 % for a-c and 50 % for d–f, respectively.
The Transformation from Kinetically Formed Hagg to JCT States
The transformation of the Hagg to the JCT-state was then studied over time by monitoring changes in the absorption spectra (Figure 2a, water content, 70 vol %). The time dependence of the absorbance at 570 nm exhibited a sigmoidal transition (Figure 2b), indicative of an autocatalytic process.11a The correlation between the temporal changes in Vis spectra and the aggregation morphology was investigated by scanning electron microscopy (SEM). Immediately after adding water to PDI-1 in THF, nanoparticles (NPs) with average diameters of 170 nm are observed (Figure 2d and transmission electron microscopy (TEM) images in Figure S9). Meanwhile, after 2 hrs, well-developed, one-dimensional helical superstructures were observed for the JCT-state (Figure 2e). The helical superstructures eventually transformed into nanotubes, as confirmed by TEM images (Figure 2f). Due to the chiral glutamine group connected to the PDI chromophore, homochirality was observed for L or D-type compounds (Figure S10). The JCT-state of L/D-PDI-1 showed right- and left-handed helices, respectively. The observed morphological changes to the regular helical superstructures are consistent with the changes in Vis and CD spectra (Figures 2a, c). The observed time-dependent spectral changes (i.e., from the H- to the JCT-state) depend on the water content, as shown in Figures S11–S14. The molecular orientation change is more rapid at lower water concentrations, taking only ∼ 20 min for a 50 % water volume fraction (Figure S11). On the other hand, the mixtures with 60 % and 70 % water content took longer, 60 min and 120 min, respectively, for the spectral changes. Except for the 90 % water sample (Figure S16), all of these samples showed the temporal change from the H- to JCT-state with morphological changes from NPs to helical nanofibers (Figure S15). These are typical molecular orientational changes from the kinetically trapped, metastable state to the thermodynamically stable state.14a, 14f, 24 The transformation from the kinetically formed aggregate Hagg to the thermodynamically stable JCT state was further confirmed by changes in the Vis spectra measured at various incubation times at the measurement temperature when the thermally generated monomer solution was cooled to ambient temperature (Figure S17, S18). We then pursued characterizations of the THF/H2O mixture with the water volume fraction of 70 %, where both the high fluorescence quantum yield and the CD intensity of the JCT-state were observed (Table S2 and Figure S8).
The spontaneous transformation from H to JCT nanostructures is accelerated by sonication. The transformation process was completed within 2 minutes of ultrasonic irradiation (Figure S19, S20). As can be seen from the SEM images in Figure S19e and Figure S21, the nanofibrils evolve from the initial nanoparticles. Based on these observations, the mechanism of aggregation and the following transformation can be considered as follows: when water is injected into the THF solution, the hydrophobic nature of the PDI core leads to the formation of nanoparticles via solvophobic interactions and π–π interactions, which kinetically determine the molecular orientation.23a As time progresses, the molecular arrangement within the H-aggregates changes, eventually giving rise to thermodynamically favorable crystalline nuclei of the JCT-state. Subsequently, a supramolecular polymerization reaction occurs on these nuclei, forming one-dimensional chiral nanofibers.
The changes in molecular orientation during the transformation process were further confirmed by Fourier transform infrared (FT-IR) spectra (Figure S22). The as-prepared and incubated samples were drop-cast on gold-coated glass, and solvents were evaporated under vacuum and then observed in reflection mode. As discussed below, different FT-IR spectra were obtained for these cast samples, which would reflect the corresponding dispersion state. The N−H stretching vibration of Hagg observed at 3380 cm−1 shifted to 3307 cm−1 in the JCT-states, indicating stronger intermolecular hydrogen bonding formed in helical JCT-nanostructures.14g, 25 CH2 stretching vibrations in JCT-nanostructures were detected at 2919 and 2850 cm−1, suggesting the crystalline packing of long alkyl chains.25b, 26 In contrast, Hagg exhibited CH2 stretching vibrations at 2923 and 2,854 cm−1 on the higher energy side, and these high wavenumber peaks imply that the alkyl chains lack crystalline order.26 These observations confirm that the helical or tubular nanoassemblies (JCT-state) have regular crystalline order,27 which is higher than that in the kinetically formed NPs (Hagg). As discussed in the former section, the Hagg in kinetically formed NPs showed exciton splitting in the CD spectra (Figure S4), indicating that the chromophores have a specifically ordered arrangement under the influence of the chirality of the glutamate unit. On the other hand, the lack of crystalline ordering of alkyl chains in NPs may be attributed to the coexistence of intramolecular hydrogen-bonded species in Hagg that disrupt the packing of alkyl chains (Figure S23).
These temporal transformation processes found for PDI-1 are also observed for PDI-2 and PDI-3, which have linear alkyl chains of carbon numbers C10 and C3, respectively (Figure 3, Figures S24–S28). Similarly to the case of PDI-1, initially formed H-aggregates with a nanoparticle morphology exhibited bisignated CD spectra. In contrast, the CD spectral intensities are significantly amplified upon transforming into the thermodynamically stable JCT-state. It is to be noted that compound PDI-2 has a saturated C10 hydrocarbon chain instead of a flexible oleyl chain as in PDI-1, and the solvophilicity (i.e., the degree of solvation) of such a saturated hydrocarbon in organic media is lower than that of the oleyl chains.28 In PDI-3, the alkyl chain length of C3 is very short in attaining solvophilicity, but its behavior in water-THF mixtures is similar to PDI-1 and PDI-2. These observations indicate that the chiral L-glutamate long-chain units containing the flexible ether and amide groups serve as solvophilic groups in the water-THF mixtures and direct the formation of the JCT-state and helical superstructures.
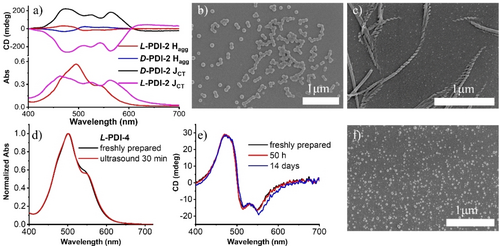
(a) CD spectra of Hagg and JCT of L/D-PDI-2. SEM images of freshly prepared Hagg (b), and JCT of L-PDI-2 (c). (d) Normalized absorption spectra of L-PDI-4. (e) CD spectra of L-PDI-4. (f) SEM image of L-PDI-4 after being treated by ultrasound for 30 min. Conditions: [c]=2.5×10−5 M, THF/H2O (3 : 7, V/V).
Structural Requirements for PDI to Show FE/CTE Coupling
In contrast, PDI-6, in which chiral long-chained glutamate units are symmetrically introduced to both ends of the PDI chromophore, only gives absorption spectra and exciton-coupled CD spectra characteristic to the kinetically trapped H-aggregates, even after incubation of 2 weeks or ultrasonication for 40 min (Figure S29). Thus, the symmetric introduction of the lipophilic L-glutamate moieties into the PDI chromophore stabilizes the kinetically formed Hagg. The transformation of the JCT state observed for PDI-1, PDI-2, PDI-3 is also absent in those with branched alkyl chains - PDI-4, PDI-5, and PDI-7. These PDIs with branched alkyl chains only formed Hagg, even sonicated in aqueous THF (50 % v/v), and inhibited conversion to JCT-states (Figure 3d–f, Figures S30–S33). These observations indicate that the asymmetric introduction of linear alkyl chains to PDI chromophore is essential to show the distinct self-assembly pathway (Hagg to JCT-state).
In general, self-assemblies of asymmetric molecules, such as amphiphilic molecules, adopt symmetric aggregation structures because they are stable on the basis of statistical thermodynamics.29 For example, in the aqueous self-assembly of lipids, bilayer structures with the hydrophobic alkyl chain end facing each other at the middle of bilayers are formed. Although detailed information on the alignment of PDI molecules in the current water-THF mixtures is unavailable, it is natural to assume that they give entropically favorable symmetric molecular assembly structures with respect to the inversion operation.29
To provide clues to understanding the differences in self-assembly behavior observed in linear and branched alkyl chain compounds, the substructures near the PDI chromophore were optimized by DFT calculations (Figure S34). Two alkyl chains linked to the glutamine residue bound to the PDI chromophore will align in these PDI assemblies. If the alkyl chains introduced on the opposite side of the PDI are linear, they will inevitably be oriented to one side of the PDI plane with a certain tilt angle (Figure S34a). The aligned linear chains in the PDI assemblies allow for slippage to displace the orientation of the PDI chromophore while maintaining proximity distance. This situation is consistent with the discussion by Spano et al., which states that the change in molecular orientation from the H-aggregate to the CT-promoted J structure can be caused by small lateral displacements between adjacent chromophores.22b On the other hand, in compounds PDI-4, PDI-5, PDI-6, and PDI-7, the branched alkyl chains extend above and below the PDI ring plane (Figure S34b-d), and they provide a steric hindrance to prevent the proximity distance of PDI chromophores required for the short-range coupling via CT interactions. Although the steric hindrance by the branched units can be alleviated by rotational displacement of the PDI chromophores, they are still H-aggregates whose orientation is unsuitable for short-range CT coupling (Figure S35). To support the above reasoning, we designed and synthesized another PDI compound PDI-8, which contains an isobutyl group (Figure S36). In this case, as with the linear PDI compound, the structure was optimized with the isobutyl group oriented on one side of the PDI plane. However, the spontaneous transformation from Hagg to JCT-states was inhibited. On the other hand, it occurred very slowly after adding nanocrystalline seeds (Figure S36c), indicating that the branched isobutyl group also provides resistance to molecular reorientation. These observations underline that linear alkyl chains are optimal for smooth molecular reorientation to JCT-states. The following section discusses the acceleration of transformation by nano-seeds in detail.
Living Supramolecular Polymerization of JCT-States
Interestingly, JCT-nanostructures formed by the asymmetric, chiral PDIs showed seed-induced living supramolecular polymerization. The seed nanocrystals of L-PDI-1 were prepared by ultrasonication of the H-aggregate dispersion for 15 min. This ultrasound treatment led to the aggregate fragmentation into shorter twisted fibers (Figure S37). We note that this process did not alter the optical properties compared to the JCT self-assemblies formed via spontaneous transformation (Figure S38). When 0.05 or 0.1 equiv. (molar ratio) of nanoseeds was added to the freshly prepared dispersion of L-PDI-1, an immediate change from Hagg to JCT-nanostructures was observed at ambient temperature without lag time (Figure 4a, Figure S39). Moreover, the time required for complete conversion into the thermodynamically stable JCT-nanostructures was shortened to 20 minutes or 6 minutes after the addition of the nano-seeds (5 mol % and 10 mol % respectively (Figure 4, Figure S39), which are much faster than the spontaneous transformation (typically 120 minutes).
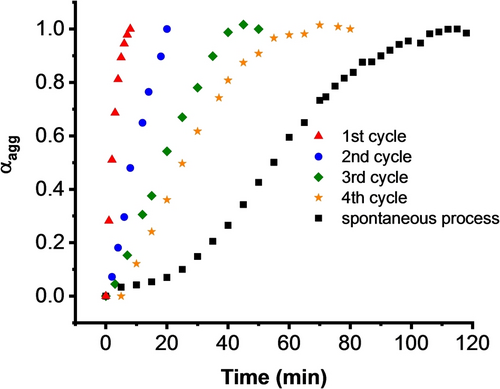
Plots of the degree of aggregation (αgg) of L-PDI-1 calculated from the extinction coefficient at 570 nm after adding 0.1 equiv. seed.
Subsequently, we carried out a consecutive polymerization process (Figure S40), i.e., utilizing the supramolecular polymer obtained from the first polymerization cycle as the crystalline nano-seeds to promote 2nd polymerization, and the same procedure was repeated to a fourth cycle. When 10 mol % of the nanocrystalline seed from the first cycle was added to the freshly prepared solution of L-DPI-1, polymerization began immediately, although a more extended period of approximately 20 minutes was required to complete the transformation (Figure 4). The 3rd and 4th cycles also showed an accelerated polymerization process compared with the spontaneous process but with slower polymerization rates. These results indicate essential features in supramolecular polymerization: (1) The nano-seeds act as nuclei for the polymerization process in a living manner, and (2) although the ends of the supramolecular polymer remain active, the transformation rate is diminished due to a reduction in the number of active ends after the last seeded process.11a, 14b This phenomenon is evident in the elongation of nanofibers after each seeded process (Figure S37 and S41). The seed-induced living supramolecular polymerization processes were also observed for L-PDI-2 and L-PDI-3 (Figures S24d and S27d, respectively). Furthermore, hetero-seeded polymerization processes can also be achieved for L-DPI-1, L-DPI-2, and L-DPI-3 (Figure S42). These results demonstrate that nanocrystalline seeds promote the transformation from H-aggregate to JCT-nanostructures compared to the spontaneous process, and JCT-nanoassemblies show living supramolecular polymerization.
We then applied the hetero-seeded polymerization process to the branch-chained compounds L-PDI-4, L-PDI-5, L-PDI-6, and L-PDI-7. When 10 mol % of nano-seeds obtained from L-PDI-3 was added to the freshly prepared samples of L-PDI-4, L-PDI-5, L-PDI-6, and L-PDI-7, no significant changes were observed, as shown in Figure S43. These results indicate that the branched chain-substituted PDIs are inert to the nanocrystalline seed of L-PDI-3 and do not show pathway complexity30 because of the considerable difference in molecular structures and alignments. Thus, nanocrystal-induced living supramolecular polymerization occurs specifically for asymmetric, linear-alkyl chain-having PDI molecules that form JCT-nanostructures.
Fluorescence and CPL Characteristics of JCT-Assemblies
The significant exciton interactions in JCT-nanostructures prompted us to study the relationship between molecular organization, fluorescence, and circularly polarized luminescence in PDI-1.12a, 31 PDI-1 is monomerically dissolved in pure THF (Figure 1a, b), which gave a high fluorescence quantum yield Φfl of 69.7 % and a lifetime of 5.2 ns at λem=535 nm. These values are consistent with the previous report for PDI chromophores.32 On the other hand, the fluorescence of π-stacked PDI chromophores is generally significantly quenched. Consequently, quantitative evaluation of radiative and nonradiative decay channels in molecular assemblies has rarely been performed. The obtained fluorescence Φfl and lifetimes for kinetically formed Hagg and thermally stable JCT-state observed at water volume fractions of 50–80 % are summarized in Table S2.
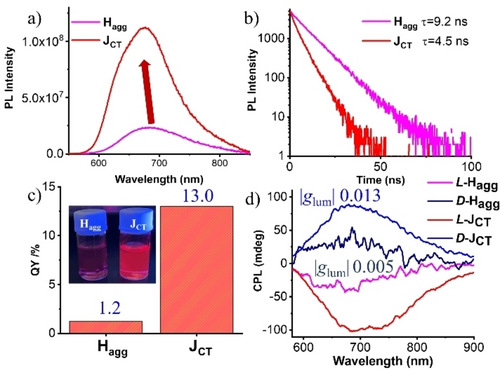
PL spectra (a), lifetime (b), the absolute emission quantum yield (c), and CPL spectra of freshly prepared Hagg and JCT-nanostructures of PDI-1(d). Conditions: [c] =2.5×10−5 M, THF/H2O (3 : 7, V/V).
The red-colored excimer fluorescence observed for Hagg at 691 nm under UV irradiation (365 nm) showed an emission lifetime τave of 9.2 ns (Figure 5a, b, Table S2). Interestingly, the JCT–state showed a 14 nm blue-shifted excimer emission at 677 nm with a shorter emission lifetime τave of 4.5 ns.
In order to unveil the contribution of radiative and nonradiative decay processes and to understand the effect of mixed FE/CTE state on fluorescence characteristics, radiative and nonradiative decay time constants for each of Hagg and JCT-state was determined by using Φfl=kr×τave and kr=1/τave–knr. We find that Hagg shows kr of 1.30×106 s−1 and knr of 1.07×108 s−1, whereas JCT-state shows kr of 2.89×107 s−1 and knr of 1.93×108 s−1, respectively. Compared to Hagg′s nonradiative decay time constant knr, that of JCT-state showed a 1.8-fold increase. The significantly increased fluorescence quantum yield Φfl observed for JCT-state by 10 times is ascribed to a more than 22-fold increase in the radiative decay constant kr. These results show for the first time that FE/CTE mixing in the self-assembly of PDI significantly increases the quantum yield and that this is due to a significant increase in the radiative decay constant kr. The enhanced radiative decay constant indicates the presence of coherent excited domains that show efficient excitonic migration in the JCT-state assembly.33
Subsequently, the excited state chirality was investigated by CPL (Figure 5d). Mirror-imaged CPL signals were observed for freshly prepared Hagg, reflecting the exciton coupling between PDI cores aligned with rotational displacement. Notably, the glum value also exhibited a remarkable increase in the course of transformation from Hagg (∼5×10−3) to JCT-nanostructures (∼1.3×10−2). These results indicate that controlled molecular orientation from kinetically trapped Hagg to thermodynamically stable JCT-nanostructures in helical superstructures enhances chiroptical properties. This is the first example of supramolecular living polymerization for the chiral FEs/CTEs coupling systems, which gave thermodynamically stable chiral molecular self-assemblies with remarkably enhanced fluorescence, CD, and CPL spectral properties.
Conclusion
In summary, we developed a family of asymmetric PDIs containing a double-chained chiral glutamate group on one end of the PDI chromophore and linear or branched alkyl chain units on the other side. The PDIs featuring a linear alkyl chain on the other end exhibited significant FE/CTE mixing and showed pathway-dependent energy landscape of self-assembly; the freshly prepared samples formed H-aggregate, which transformed into chiral JCT-nanostructures over time or by adding nanocrystalline seeds in a living manner. Meanwhile, the PDIs with branched alkyl chains did not show structural transformation into chiral nanostructures or supramolecular polymerization. The PDIs with branched achiral chains only formed H-aggregates as kinetic and thermodynamically controlled species. Thus, FE/CTE coupling and supramolecular living polymerization properties can be controlled according to the molecular design of the chiral DPIs. Compared to the kinetically obtained H aggregates, significant enhancements of the fluorescence quantum yield, CD intensity, and glum value in CPL characteristics were observed for the JCT state. We showed that the increase in quantum yield of the JCT state is attributed to the increase in radiative decay constant kr, indicating the presence of a coherent FE/CTE hybrid exciton domain; this work provides valuable insights into developing FE/CTE hybrid exciton materials with high-performance luminescence and chiroptical materials that show supramolecular living polymerization. The concept of living hybrid exciton materials developed in this study will contribute to the design of photo-functional materials from a new perspective.
Supporting Information
The data that support the findings of this study are available in the Supporting Information of this article.
Acknowledgments
The authors thank the financial support of the JSPS KAKENHI grant numbers JP20H05676, JP22KF0294, and JP20H02781. H.J. acknowledges the JSPS postdoctoral fellowship for foreign researchers.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.