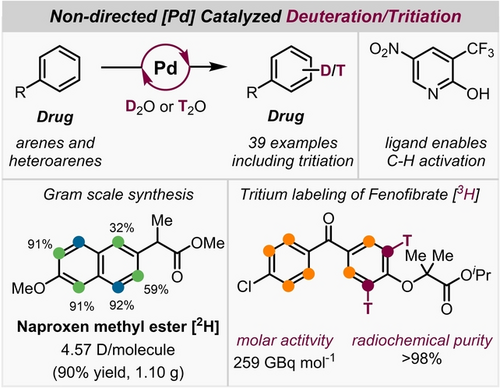
Deuteration and Tritiation of Pharmaceuticals by Non-Directed Palladium-Catalyzed C−H Activation in Heavy and Super-Heavy Water
Graphical Abstract
We present a hydrogen isotope exchange reaction using non-directed homogeneous Pd-catalysis. The catalytic system relies on a commercially available pyridine ligand that enables aromatic C−H activation. Labelling of 39 pharmaceuticals was achieved by utilizing D2O as the deuterium source and solvent, without requiring fluorinated cosolvents. Expansion to T/H exchange on three different pharmaceuticals by using T2O as isotopic source was demonstrated.
Abstract
Deuterated and tritiated analogs of drugs are valuable compounds for pharmaceutical and medicinal chemistry. In this work, we present a novel hydrogen isotope exchange reaction of drugs using non-directed homogeneous Pd-catalysis. Aromatic C−H activation is achieved by a commercially available pyridine ligand. Using the most convenient and cheapest deuterium source, D2O, as the only solvent 39 pharmaceuticals were labelled with clean reaction profiles and high deuterium uptakes. Additionally, we describe the first application of non-directed homogeneous Pd-catalysis for H/T exchange on three different pharmaceuticals by using T2O as isotopic source, demonstrating the applicability to the synthesis of radiotracers.
Introduction
Interest in facile and simple exchange of protium by higher isotopes is constantly growing due to the characteristic and unique properties of the resulting labelled compounds.1 The incorporation of non-radioactive deuterium2 is utilized for a wide range of applications, exemplified by drugs deucravacitinib, deutetrabenanzine (both FDA approved, Scheme 1A) and VX-984 (completed Phase I trial).3 In heavy drugs, the primary kinetic isotope effect4 (KIE) which induces a slower C−D over C−H bond cleavage can improve the pharmacokinetic and pharmacodynamic properties of an active pharmaceutical ingredient (API). Perdeuterated compounds are also utilized as internal standards in mass spectrometry, thus making a crucial contribution in metabolomics.5, 6, 7 Facilitating synthetic access to tritiated drug candidates is also essential in drug discovery.8 3H- and 14C-labelled radiotracers are still considered to be gold standard probes in many steps of absorption, distribution, metabolism and excretion (ADME) studies.9 Deucravacitinib and deutetrabenanzine contain deuterated methyl groups next to heteroatoms to limit their metabolization rate mediated by cytochrome P450 dealkylation processes.10 Access to deuterium incorporation of (hetero-)arenes11 could offer new opportunities to improve the pharmacokinetic or toxicity profile of drugs metabolized by other enzymes such as aldehyde oxidase. The development of efficient methodologies allowing the perdeuteration of aromatic OLED components and fluorescent probes is also of high interest. For such purposes, KIE can induce a significant increase of OLED display lifetimes or reduce photobleaching phenomena.12
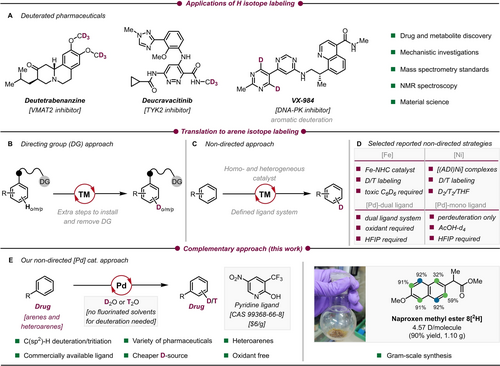
Importance of hydrogen isotope labeling and methods for labeling of arenes. NHC = N-heterocyclic carbene; ADI = α-diimine.
Existing methods to establish aromatic H/D exchange rely on use of Lewis or Brønsted acids to exploit SEAr reactivity.13 Recently, a superbasic sodium amide donor was utilized for perdeuteration.14 A wide range of heterogeneous metal catalysts have also been developed to conduct this transformation.15 Harsh reaction conditions and side product formation generally limits their applicability to only simple systems. Homogeneous catalysis offers milder and more selective alternatives. Selective deuteration in the meta- or ortho-position is achievable by a variety of developed directing group (DG) approaches (Scheme 1B).16 Nevertheless, most of these efficient methods are limited by the requirement of installing directing groups that are not necessarily cleavable.17
In contrast, unbiased labeling of simple arenes is achievable through non-directed C−H activation strategies utilizing defined ligands in combination with transition metals (Scheme 1 C). Chirik et al. managed to successfully deuterate and tritiate more complex arenes utilizing an Fe-pincer complex and D2/T2 gas as the isotopic source.18 The same group later developed Ni-catalyzed transformations which allowed D- and T-labeling.19 In 2020 the de Ruiter group reported a method utilizing an Fe-pincer complex which enables H/D exchange using liquid d6-benzene.20 Independently, Chirik employed an Fe-based system to utilize d6-benzene as the deuterium source.21
Following these findings, the research on non-directed H/D exchange focused on Pd catalysis often paired with solvents as deuterium source. In this context, Ritter and co-workers reported deuteration/tritiation of aryl thianthrenium salts with a molecular Pd catalyst utilizing D2/T2 gas.22 Van Gemmeren et al.23 applied dual-ligand systems consisting of a combination of N-acyl amino acids and N-heterocycles to deuterate a wide range of functionalized arenes in mixtures of D2O and HFIP.24 Recently, the Joo group introduced a pyrazolopyridine ligand that enabled perdeuteration of (het-)arenes in HFIP/d4-AcOH mixtures25 (Scheme 1D). Limitations of both methods included the requirement of fluorinated solvents. Per- and polyfluorinated substances in particular as solvents often bring unique reactivity.26 However, these substances are frequently banned due to their toxic nature and the production of non-degradable waste.27 Herein, we present a scalable method that tolerates differently functionalized pharmaceuticals and drug scaffolds for H/D exchange without the need for additional fluorinated solvents (Scheme 1E). Pd-catalyzed labeling is achieved by the use of D2O or T2O in combination with a cheap and commercially available 2-pyridone ligand.28 This ligand class plays a crucial role in non-directed C−H functionalizations due to unique properties which were shown by several research groups.29, 30 We envisioned the establishment of a homogeneous non-directed method for late-stage hydrogen isotope exchange (HIE) by use of a Pd-pyridone catalyst to achieve reversible C−H activation in drugs. A further focus was put on using D2O as the isotopic source and to avoid fluorinated solvents.
We initiated our investigation by studying deuteration of 1,2-dimethoxybenzene as a model substrate utilizing D2O and 2-hydroxy-pyridine as a ligand. In the presence of Pd(OAc)2 the reaction resulted in moderate H/D-exchange (Table S1, entry L1, see Supporting Information). Based on these observations, we decided to evaluate various 2-pyridone ligands in combination with our model substrate. We observed that the electron-deficient pyridones delivered moderate to good deuterium incorporation at both the α and β positions of 1,2-dimethoxybenzene (Table S1, entries L2, L3, etc., see Supporting Information). After a thorough screening, the highly electron-deficient 5-nitro-3-(trifluoromethyl)-pyridin-2-ol (L19, see Scheme 2) was found to be optimal for incorporating deuterium in higher degrees. Electron-rich pyridines acted inefficiently in this reaction. We believe a similar mode of action is operating, as it has been proposed before in the literature, where one pyridone binds in a bidentate fashion and another serves as an internal base to promote concerted metalation and deprotonation.29 Regioselectivity is determined by both electronic and steric properties of the substrate. This pyridone ligand, along with accelerating the reaction rate, also prevents decomposition of the catalyst, hence increasing the catalytic efficacy. Other more expensive or toxic deuterated solvents such as d2-HFIP and d4-AcOH, or combinations thereof showed decreased degrees of D incorporation (Table S2, see Supporting Information). Control experiments revealed that both Pd-catalyst and ligand are essential to obtain optimal results (Table S6, see Supporting Information). After a diverse screening, Pd(OAc)2 as catalyst, ligand L19, 110 °C, and a reaction time of 48 h in D2O as the only solvent were found to be the best reaction conditions. Additives (e.g. oxidants) did not lead to better results. To predict the reversibility of the reaction, we subjected [D]-1,2-dimethoxybenzene to our optimized reaction conditions using H2O as the solvent. A significant amount of [H] incorporation was observed, which illustrates that the C−H activation step is reversible (see Supporting Information).
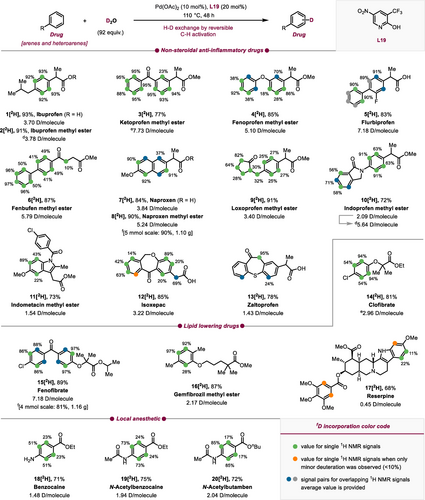
Deuteration of pharmaceutical compounds.a,b,c,d,e,f,g aReactions were performed on a 0.3 mmol scale. bDeuterium incorporation was determined by 1H NMR (integrated relative to unlabeled protons in the same compound), cYields were determined by 1H NMR of the crude reaction mixture using 1,3,5-trimethoxybenzene or dibromomethane as an internal standard. dSecond cycle using already deuterated material under standard conditions. eReaction at 120 °C. fSee Supporting Information for deuterium degrees. g1H NMR signals which are not baseline separated are reported as signal pairs with average degree of deuteration; magnetically equivalent positions are labeled as single signals (green; for experimental details, see Supporting Information).
With optimized conditions for our test substrate in hand, we began by applying our deuteration protocol to nonsteroidal anti-inflammatory drugs (NSAID, Scheme 2), a class of pharmaceuticals that is extensively utilized to alleviate pain associated with various conditions, particularly arthritis. We first tested the deuteration of the free acid ibuprofen (1) and its methyl ester (2) which resulted in excellent H/D exchange (3.70 and 3.78 D/molecule). Conversion of ketoprofen methyl ester 3 led to the corresponding deuterated product 3[2H] with high D incorporation (7.73 D/molecule) and in excellent yield. Employing our conditions for other commercially available NSAIDs resulted in significant deuteration of fenoprofen methyl ester (4) (5.10 D/molecule) and flurbiprofen (5) (7.18 D/molecule) which contains a free carboxylic acid. Notably, this represents the first reported perdeuteration of flurbiprofen. The biaryl containing fenbufen methyl ester (6) showed a high level of deuterium incorporation (5.79 D/molecule). Our protocol also enables access to perdeuterated naproxen 7[2H] and naproxen methyl ester 8[2H]. Previous labeling by Gannett et al. delivered naproxen deuterated in C1, C4, and C7 positions.31 Loxoprofen methyl ester (9), which features a cyclopentyl core, exhibited noteworthy deuterium incorporation not only in the aromatic core but also in the alkyl protons adjacent to the α-keto position. This observation suggests the involvement of an acid/base-type mechanism. Indoprofen methyl ester (10) and indometacin methyl ester (11) which contain isoindolin and indole cores, performed well when subjected to the established protocol. Other NSAIDs that contain heterocyclic cores, such as isoxepac (12) with a dihydrodibenzoxepin group, and zaltoprofen (13) with a dihydrodibenzothiepin structure were deuterated in high degrees (3.22 and 1.43 D/molecule). Next, we turned our focus to lipid-lowering drugs which are employed to regulate elevated levels of cholesterol and triacylglyceride in the bloodstream. Conversion of Clofibrate (14), which contains an aryl halide function, led to its deuterated version 14[2H], (2.96 D/molecule, Scheme 2). Fenofibrate (15) showed a remarkably high level of deuterium incorporation (7.18 D/molecule) compared to previous literature reports.24, 25 Additionally, gemfibrozil methyl ester (16) afforded high levels of deuterium uptake (2.17 D/molecule). No proto- or deuterodepalladation was observed for aromatic halides. Reserpine (17), a drug with a complex polycyclic heteroaromatic structure, exhibited a low level of deuterium incorporation. This is likely attributed to the complexity of its sterically demanding structure which can hinder catalyst binding. In addition, we employed our protocol for local anaesthetic drugs such as benzocaine (18, free amine), N-acetylbenzocaine (19), and N-acetylbutamben (20) to synthesize the deuterated derivatives 18[2H], 19[2H], and 20[2H].
Through repetition of the reaction using already labelled scaffolds 2[2H] and 10[2H] (Scheme 2), we successfully increased the level of deuterium incorporation compared to the first deuteration. This is highlighted by the increase from 2.94→3.78 for 2[2H] and 2.09→5.64 D/molecule for 10[2H]. This shows the potential of our method to achieve higher degrees of deuteration by iterative conversions. Furthermore, the reaction was expanded to gram scale (4.0 and 5.0 mmol) with similar levels of deuterium labeling achieved for naproxen methyl ester 8[2H] (4.57 D/molecule, 90 %, 1.10 g) and fenofibrate 15[2H] (3.10 D/molecule, 81 %, 1.16 g). Combination of scale-up reactions and iterative conversion can be used to obtain gram quantities of highly deuterated material.
Next, we evaluated various other drug molecules and natural products with diverse functional groups (Scheme 3). Nefiracetam (21) showed a substantial level of deuteration in excellent yield (2.13 D/molecule). Deuterated d9-caffeine has already been utilized in a variety of biological studies and clinical trials for various treatments.10 Encouraged by the lack of existing methods to prepare deuterated caffeine derivatives, we have successfully accomplished a substantial H/D exchange on the arene moiety, resulting in the synthesis of d1-caffeine 22[2H]. Herniarine (23) a methoxy derivative of coumarin reacted with high D incorporation. Desired H/D exchange was achieved on phenolic vanillin (24, 2.46 D/molecule). In guiazulene 25[2H] the position with the highest steric congestion displayed a relatively limited level of H/D exchange (total of 1.26 D/molecule). On the other hand, δ-tocopherol-OMe (26,Vitamin E) and watermelon ketone (27) both displayed good degrees of deuteration when subjected to the protocol. H−D exchange in watermelon ketone (27) additionally took place on the methyl unit and in α-positions to the ketone. Conversion of steroidal O-acetylestrone (28) provided high values of deuterium enrichment (1.89 D/molecule). Conversion of piperine (29) resulted in a moderate level of deuterium incorporation on the arene moiety and in the olefinic positions. Alongside drugs and natural products, our protocol also proved to be applicable to fungicides. We conducted reactions with sedaxane (30) and benzovindiflupyr (31), molecules that both contain pyrazole-4-carboxamides motifs, and found significant deuterium enrichment. Additionally, metalaxyl (32) and etofenprox (33) reacted smoothly. The oxazolidine-dione compound famoxadone (34) was perdeuterated in only low degrees. The final substance class we explored was simple heterocycles, common motifs in pharmaceuticals. However, their ability to coordinate to TM complexes can lead to reduced catalytic activity. Pyrrole derivative 35 and benzothiophene derivative 36 were both converted with high degrees of deuteration and in good yields (35[2H] 3.09, 36[2H] 3.87 D/molecule). Furthermore, indole derivatives 37 and 38 were well tolerated under our protocol. In addition, N-pivaloylindole (39) gave excellent H/D exchange (5.58 D/molecule). Compared to previous works,24, 25 for drug candidates such as ketoprofen methyl ester (3), fenbufen methyl ester (6), naproxen methyl ester (8), clofibrate (14), fenofibrate (15), and watermelon ketone (27) similar results in terms of D incorporation and yields were obtained. Recently reported HIE methods based on non-directed Pd catalysis were not applied for tritium labeling.24, 25 The use of HTO as isotopic source for such purposes is considered to be more delicate than the use of T2 gas. Nevertheless, given the excellent functional group tolerance of this method, we envisioned exploring T-labeling to synthesize potential radiotracers by the use of HTO. In this context, using fenofibrate (15, Scheme 4) as a substrate, we optimized the reaction conditions with the aim of minimizing the amount of isotopic source while furnishing a reasonable isotopic enrichment and a clean reaction profile. As a result, using ~5 eq of tritiated water generated by the reduction of PtO2 in a TFT/dioxane mixture, tritiated fenofibrate (see Supporting Information) was obtained with a molar activity of 259 GBq mol−1 and high radiochemical purity (>98 %) after a simple filtration of the crude mixture through a celite pad. T-labeling sites of fenofibrate 15[3H] were assigned by 3H NMR experiments. Additional deuterium experiments (see Supporting Information) using a low amount of isotopic source suggested that the isotopic enrichment can be significantly increased using higher amounts of the Pd-ligand system and have confirmed the pivotal role of the latter in the isotope incorporation.
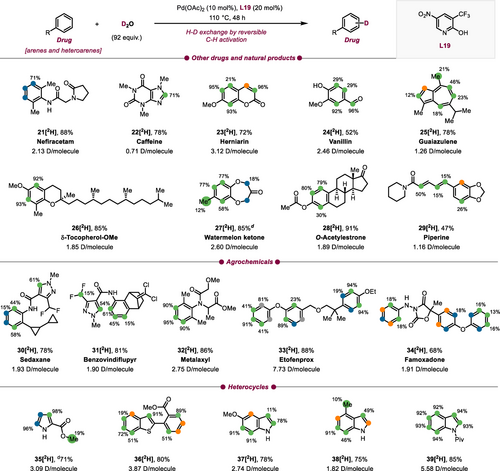
Deuteration of drugs, natural products, agrochemicals and heteroarenes.a,b,c,d,e aReactions were performed on a 0.3 mmol scale. bDeuterium incorporation was determined by 1H NMR (integrated relative to unlabeled protons in the same compound), cYields were determined by 1H NMR of the crude reaction mixture using 1,3,5-trimethoxybenzene or dibromomethane as an internal standard. dIsolated yield. e1H NMR signals which are not baseline separated are reported as signal pairs with average degree of deuteration; magnetically equivalent positions are labeled as single signals (green; for experimental details, see Supporting Information).
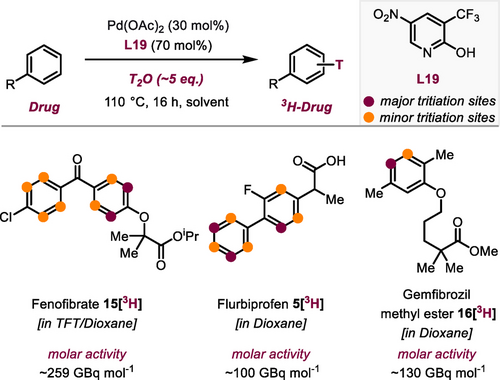
Synthesis of radiolabeled pharmaceuticals by palladium catalyzed C−H tritiation using HTO as isotopic source. TFT= (trifluoromethyl)benzene.
Using reaction conditions similar to those used for the synthesis of fenofibrate 15[3H], but whithout fluorinated solvent, tritiated analogues of flurbiprofen 5[3H] and gemfibrozil methyl ester 16[3H] were synthesized (Scheme 4). 5[3H] and 16[3H] were obtained with molar activities reaching ~100 GBq/mmol and ~130 GBq/mmol, respectively, and satifactory radiochemical purity after a simple filtration through a silica pad. Overall, these results demonstrate the applicability of such a methodology to synthesize radioactive analogues of drug candidates usable for ADME studies of APIs.
Conclusion
In summary, we have developed a versatile protocol for the non-directed Pd-catalyzed C−H deuteration and tritiation of aromatic pharmaceuticals utilizing a commercially available pyridine ligand. This method is applicable to both electron-rich and electron-poor aromatic compounds and is compatible with a wide range of functional groups, making it a valuable method that complements existing protocols.24, 25 In contrast to existing methods this protocol only relies on D2O as solvent and isotopic source and does not require fluorinated solvents. In the context of the synthesis of tritiated analogues of API, the method described may offer an interesting alternative to well established tritiation protocols particularly in terms of radioisotope incorporation regioselectivity. We expect that the accessibility of highly deuterated and tritiated compounds through this method will facilitate accelerated and extensive exploration of the biological activity of labelled molecules.
Supporting Information
The authors have cited additional references within the Supporting Information.32-34
Acknowledgments
Financial support from the Indian Science and Engineering Research Board (SERB) (CRG/2022/004197 to D. M.) and Department of chemistry, Indian Institute of Technology Bombay (IPDF to C.T.) is gratefully acknowledged. D. M. gratefully acknowledges the Alexander von Humboldt Foundation and the Freiburg Institute of Advanced Studies (FRIAS) for funding his stay at the University of Freiburg. We thank Gwyndaf A. Oliver for proofreading the manuscript. G.P. and P.C. warmly thank Sébastien Garcia Argote for his crucial help in conducting the tritium labeling experiments, and Timothée D′Anfray and David Buisson for their analytical support. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.