Total Synthesis of (±)-Baphicacanthcusine A Enabled by Sequential Ring Contractions
Graphical Abstract
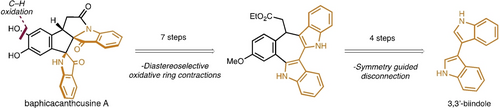
An oxidative ring contraction strategy was employed to accomplish the first total synthesis of baphicacanthcusine A. Rapid construction of a key seven-membered-ring intermediate set the stage for a series of diastereoselective oxidations to access the skeleton of the natural product. Selective C−H oxidation gave rise to baphicacanthcusine A in 11 steps from 3,3’-biindole.
Abstract
Reported herein is the first total synthesis of the poly-pseudoindoxyl natural product baphicacanthcusine A. The synthesis leverages the oxidative rearrangement of indoles to pseudoindoxyls to install vicinal pseudoindoxyl heterocycles in a diastereoselective manner. Key steps include an acid-mediated cyclization/indole transposition, two diastereoselective oxidative ring contractions, and a site-selective C−H oxygenation. The synthesis of the oxidation precursors was guided by recognition of an element of hidden symmetry. This work provides a foundation for the chemical synthesis of other poly-pseudoindoxyl alkaloids.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.