Dissociative Electron Attachment Dynamics of a Promising Cancer Drug Indicates Its Radiosensitizing Potential
Graphical Abstract
2-Bromo-1-(3,3-dinitroazetidin-1-yl)ethan-1-one (RRx-001) has been suggested as a potential radiosensitizer. Here we explored the decay of the molecule upon electron attachment. The main dissociation processes of the formed anion are characterized by loss of either a bromine atom or a NO2 group, both competing for the excess electron. The fast chemistry observed may also indicate an alternative avenue for oxygenation of hypoxic tumor cells.
Abstract
2-Bromo-1-(3,3-dinitroazetidin-1-yl)ethan-1-one (RRx-001) is a hypoxic cell chemotherapeutics with already demonstrated synergism in combined chemo-radiation therapy. The interaction of the compound with secondary low-energy electrons formed in large amounts during the physico-chemical phase of the irradiation may lead to these synergistic effects. The present study focuses on the first step of RRx-001 interaction with low-energy electrons in which a transient anion is formed and fragmented. Combination of two experiments allows us to disentangle the decay of the RRx-001 anion on different timescales. Sole presence of the electron initiates rapid dissociation of NO2 and HNO2 neutrals while NO2− and Br− anions are produced both directly and via intermediate complexes. Based on our quantum chemical calculations, we propose that bidirectional state switching between π*(NO2) and σ*(C−Br) states explains the experimental spectra. The fast dynamics monitored will impact the condensed phase chemistry of the anion as well.
Introduction
Here (AB)*− represents the temporary negative ion (TNI), also called resonance, which is initially formed by the attachment of an electron matching the energy of the TNI state.4 This initial transition occurs on femtosecond timescales. The TNI may decay in A− and B which stand for the formed fragment anion and the corresponding neutral species, respectively. This dissociation process competes with spontaneous emission of the excess electron (autodetachment) as well as intra-molecular vibrational redistribution (IVR) of the energy deposited by excess electron.5 IVR, if available, may lead to stabilization of the TNI towards microsecond timescales. Anions decaying on this timescale (metastable anions) are particularly interesting for mass spectrometry since the detection window of anions opens here.6, 7 On the other hand, this fact means that it is very challenging to study the dynamics of TNI formation and DEA on earlier timescales. Pulsed radiolysis coupled with transient absorption spectroscopy has been a successfully applied experimental technique to probe the formation of anions and their decay in bulk solution on fast time scales.8, 9 In terms of experiments in the gas phase, pump-probe photoelectron spectroscopy of anions was used to gain insight into the formation and decay of TNIs.10 To mimic the initial electron attachment in such studies, electron transfer by photoexcitation of anionic iodide in a cluster was exploited.11 From a theoretical point of view, the coupling of resonant states in anionic molecular systems in the gas phase raised strong interest.12 Such coupling schemes may also enable efficient dissociation of the formed TNI.13, 14
A potential candidate for efficient DEA is 2-bromo-1-(3,3-dinitroazetidin-1-yl)ethan-1-one (C5H6BrN3O5, RRx-001, here further denoted as RRx). The molecular structure of the compound, which includes an acyl-bromide and a geminal dinitroazetidine group, is shown in Figure 1. Previous radio-biological studies suggested that RRx could be a promising cancer therapeutic as a stand-alone drug as well as hypoxic tumor cell radiosensitizer.15, 16 This energetic heterocyclic dinitroazetidine compound was derived from 1,3,3-trinitroazetidine, which found initial application as a fuel in aerospace.16 So far, RRx has been studied up to phase III clinical trials for the treatment of multiple solid tumors as well as a supportive care drug.17 Besides its potential for radiotheranostics, where it is exposed also to low-energy secondary electrons formed during irradiation of biological matter,18 RRx is a highly interesting compound from the chemical perspective. The two NO2 groups and a bromine atom (Figure 1) can compete for the incoming electron by their high electron affinities of 2.27 eV19 and 3.36 eV,20 respectively (experimentally determined values).
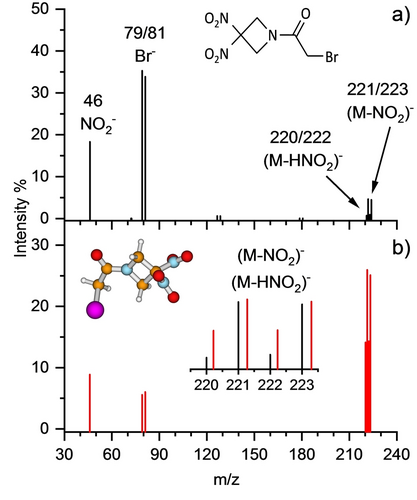
DEA mass spectra of isolated RRx (denoted as M in the two panels) after electron attachment from a) Wippi experiment, tdet~300 μs and b) CLUB experiment, tdet ~10 μs. Relative intensities were obtained by integrating raw mass spectra for different electron energies from ~0–9 eV. The inset shows relative change of intensities for (RRx−NO2)− and (RRx−HNO2)− fragment anions by comparing them scaled to the same maximum in the zoomed region. Panel a) shows the skeletal formula of the RRx molecule and in panel b) the structure obtained at the B3LYP/aug-cc-pVTZ level is included. Color code: hydrogen—grey, carbon—brown, nitrogen—blue, oxygen—red, bromine—violet.
In this study, we investigated the attachment of a free electron to RRx in the gas phase. Using experimental electron attachment spectroscopy combined with mass spectrometry and computational modeling employing Gaussian21 and Molpro22 software packages for single and multi-reference calculations, respectively, we follow here the mentioned competition on the fast timescale. In addition, we also explore the dissociation dynamics of RRx on longer timescales up to a few hundred microseconds and demonstrate that the molecule effectively decomposes into reactive anionic and neutral species.
Results and Discussion
The negative ion mass spectra of RRx measured with two different experimental setups having different detection times are shown in Figure 1. The spectra show the resulting intensities of the different anions formed as a function of the m/z. The spectrum shown in Figure 1a was measured with the Wippi apparatus (quadrupole mass spectrometer) having a considerably longer detection time of tdet~300 μs compared to the CLUB apparatus employing a time-of-flight (TOF) mass spectrometer. For the latter setup, the detection time is ~10 μs. The corresponding TOF spectrum is shown in Figure 1b. Since electron attachment is a resonance process, the intensities in the plots were obtained by integrating raw spectra at different electron energies. Also at a few microsecond detection times, no molecular anion with the expected main isotope peaks at m/z 267 and 269 is observed. However, comparing with the spectrum at longer timescales in Figure 1a, the ratio between lower- and higher-mass fragment anions is substantially changed here: (RRx−NO2)− and (RRx−HNO2)− dominate in the spectrum, while Br− and NO2− are less intense. The combined view of the Wippi and CLUB data indicates that the dissociation dynamics is not yet completed on the early microsecond time scale. In detail, the heavier anions (RRx−NO2)− and (RRx−HNO2)− may be metastable and decay into Br− and NO2− fragments. We can also observe within these two-step dissociation processes that (RRx−HNO2)− decays faster than (RRx−NO2)− since the abundance at m/z 220 and 222 is reduced more in comparison to m/z 221 and 223 at longer times (see inset in Figure 1b). We also note that Br− is eventually produced in much higher amounts than NO2−.
The dissociation products observed in the mass spectra can be interpreted based on the calculated energetics shown in the reaction scheme in Figure 2. If the anion follows the σ*(C−Br) pathway, Br− most probably leaves the molecule upon pre-dissociation. Within the π*(NO2) pathway, there are several reaction channels possible. The NO2 moiety might leave the ion directly with the reaction energy of −0.66 eV, forming an ion at m/z 221 that might further dissociate to produce NO2− in an almost thermoneutral reaction (0.01 eV). Alternatively, the NO2 moiety might roam in the vicinity of the (RRx−NO2) fragment, accepting a hydrogen atom from a CH2 group to form a neutral HNO2 molecule that leaves the ion in an exothermic reaction (−0.92 eV). The resulting ion at m/z 220 might dissociate further to form Br−, again in an almost thermoneutral reaction (0.04 eV). The singly occupied molecular orbital (SOMO) in the (RRx−HNO2)− anion at m/z 220 indicates that a non-covalent type of anionic complex is formed, with a C−Br distance of 2.80 Å and charge mainly localized on Br (−0.59 e). Such an exotic complex was computationally predicted first time for intact bromo-substituted nucleobase anions in the gas phase and the solution.23 While for these compounds the DEA reaction leading to Br− release was predicted to be substantially exothermic (>2 eV) in solution, the overall reaction energy in the gas phase was about +0.4 eV. Later mass spectrometric studies with 8-bromoadenine confirmed the existence of such molecular anion complex.24 In the present study, non-covalent type of anionic complex can be detected on mass spectrometric timescales, but it can dissociate via Br− release with the reaction energy close to 0 eV (see Figure 2). A similar complex is also formed in the case of C−Br pre-dissociation at −1.67 eV. There, the C−Br bond is 3.02 Å long. It is worth noting that recent studies demonstrate that these non-covalent anions, if present, determine the TNI dissociation dynamics in solution.25 The (RRx−NO2)− complex has a more compact structure with covalent bonds to both NO2 and Br. For this intermediate, the energetics (−0.48 eV) and charge localization on NO2 set NO2− release as the primary dissociation channel.
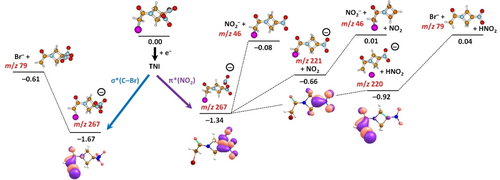
Suggested main reaction pathways upon electron attachment to RRx-001 calculated at the CCSD/aug-cc-pVDZ//B3LYP/aug-cc-pVDZ level, energies are given in eV with respect to the minimum of the neutral molecule. Singly occupied orbitals and the highest-occupied orbital of (RRx−NO2)− are shown as calculated at the B3LYP level. See the Supporting Information (Figure S1) for higher-lying dissociation channels and the (RRx)− structure formed upon electron attachment without pre-dissociation.
Figure 2 indicates that the lowest-lying DEA pathways considered in the calculations would be exothermic or nearly exothermic for all four abundant fragment anions found in the experimental spectra. Along the pathways, no significant barriers are present, which would hinder the dissociation by electrons having an initial kinetic energy of nearly zero eV. This computational prediction is supported by the single negative ion mass spectra at various electron energies which is shown in the Supporting Information (Figure S2). The ion intensities found in the negative ion mass spectrum at zero eV clearly point out that all discussed fragments are formed at this electron energy at their utmost intensity. At the incident electron energy of zero eV, the excess energy of TNI is sufficient to induce the dissociation. For some pathways, also molecular rearrangement delivers additional energy for decisive bond cleavage. In this context, it should be also mentioned that in the Wippi experiment, the molecules sublimed in the oven are directly transferred to the interaction region with the electron beam, while in the CLUB experiment, the molecules are sublimed and co-expanded with He gas into the vacuum. In the latter case, they can undergo cooling of translational but also vibrational degrees of freedom which may extend the lifetime of the anion or leads to closing of the endothermic dissociation channels. These effects will influence the relative abundance of the product anions in the mass spectra shown in Figure 1.
The experimental and theoretical observations presented so far point out the strong competition of the electron affine NO2 group and the bromine atom, competing for the negative excess charge. We also looked at this competition during the formation of the TNI and its early decay on fast timescales of femto- to picoseconds. Computational insight can be obtained from the semi-quantitative potential energy surface (PES) analysis shown in Figure 3b. RRx has high vertical and adiabatic electron affinities of 0.85 eV and 1.67 eV, respectively (at the CCSD/aug-cc-pVDZ//B3LYP/aug-cc-pVDZ level). Two lowest electronic states, allowing for electron attachment, correspond to an electron positioned in π*(NO2) orbitals of both NO2 groups, either in symmetric or antisymmetric combination as shown in Figure 3a. In the Franck–Condon (FC) region, they are almost degenerate at TD-CAM-B3LYP/aug-cc-pVTZ and MRCI(3,5)/6-31g* levels (difference of 0.14 and 0.04 eV, respectively), the EOM-CCSD/6-31g* predicts a larger gap of 0.51 eV, probably due to the single-reference treatment. At the MRCI level, the third valence electronic state lies about 1.4 eV above the π*(NO2) states, with the odd electron occupying the σ*(C−Br) orbital. Depending on the electronic state that is reached after the initial electron attachment step, pre-dissociation of either NO2 or Br moieties takes place. For prolongation of the C−Br distance, the σ*(C−Br) state is moving down in energy without any barrier, becoming the ground electronic state at the C−Br distance of about 2.2 Å; eventually, the charge of Br reaches −0.66 e. For dissociation along the C−NO2 coordinate, we also obtained a barrierless pathway from the structure of the neutral molecule, keeping the π*(NO2) character of the electronic ground state. When reaching a minimum of the pre-dissociated state, the charge on the loosely bound NO2 moiety is −0.38 e. Both pathways can result in transient parent anion structures with NO2 or Br moieties loosely bound to a polarized co-fragment, which further decay as shown in Figure 2.
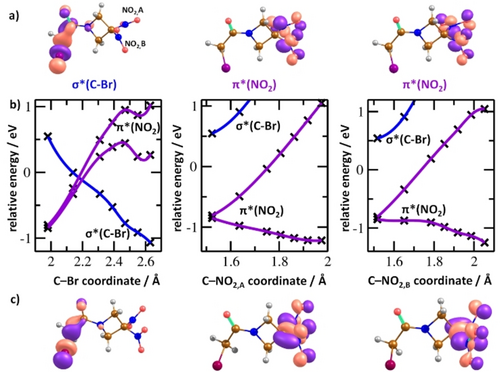
a) Orbitals occupied by the odd electron for three lowest-lying valence electronic states of the RRx anion as obtained at the TD-CAM−B3LYP/aug-cc-pVTZ level. Color code: hydrogen—grey, carbon—brown, nitrogen—blue, oxygen—red, bromine—violet. b) Interpolation curves in the RRx anion along C−Br and C−NO2 coordinates for the lowest three electronic states of valence character between the structure of the neutral RRx-001 molecule and pre-dissociation transition states (as optimized at the B3LYP/aug-cc-pVDZ level). Crosses show points calculated at the MRCI(3,5)/6-31g* level, splines are added to guide the eye. The energy is given with respect to the ground state of the neutral molecule as obtained by setting the energy of the anion in the minimum of the neutral molecule to negative of the vertical electron affinity calculated at the CCSD/aug-cc-pVDZ level (0.85 eV). c) Orbitals occupied by the odd electron for the last points of the respective interpolations in b) at the CAM-B3LYP/aug-cc-pVTZ level.
The interpolation curves of PES present in Figure 3b also show conical intersections between π* and σ* states, which would allow for state switching during the fast dissociation. As shown for less complex systems without pronounced local minima on the PES surface, a coupling of π* and σ* states can result in complex transient anion dynamics.14 Using the Wippi apparatus, we measured the anion efficiency curves of the four major anions formed upon DEA to RRx, NO2−, Br−, (RRx−HNO2)− and (RRx−NO2)−. The corresponding anion yields for the different fragments are presented in Figure 4. All fragment anions exhibit a peak near zero eV, as expected by the energetics of the dissociation pathways shown in Figure 2. Scaling all four anion efficiency curves to the zero eV feature reveals a striking difference of the ion yields: The NO2− and Br− ion yields show a pronounced tail of the first peak which is missing for (RRx−HNO2)− and (RRx−NO2)−. The anion efficiency curves of the latter two anions can be fitted by a single asymmetric peak, while the fits of the experimental NO2− and Br− data identify a second resonance in the broad tail of the zero eV peak (all fits are shown in the SI, Figure S4). The second resonance leading to NO2− and Br− is found at 0.52 and 0.56 eV, respectively. Since the corresponding thresholds of these resonance features are below the threshold of higher-lying dissociation channels (≥0.47 eV, see Figure S1), the reaction scheme shown in Figure 2 still applies. However, the difference to the zero eV peak is found in the fast TNI dynamics since we assign this yield to the initial electron attachment into the σ*(C−Br) orbital (based on the interpolation curves shown in the left panel of Figure 3b). While for predissociation of Br− the excess electron just needs to remain in this orbital, NO2− formation requires state switching at the conical intersection. Regarding (RRx−HNO2)− and (RRx−NO2)−, their anion efficiency curves suggest therefore that both anions just form by the initial occupation of the π*(NO2) orbital without involvement of the σ*(C−Br) orbital on the fast timescale. We also note that the resonance near 0.5 eV is about a factor 3 broader compared to the narrow contribution near zero eV. The width of the resonance may provide basic information about the temporary negative ion state involved. The longer life-time of π * resonances compared to σ * resonances is associated to a smaller width of the former.26 Thus, the contribution at about zero eV indicates involvement of a π * state since it nearly reflects the energy distribution function of the electron beam. The narrow width and the high intensity of the zero eV contribution indicate an s-wave electron attachment process in which the incoming electron is captured with a cross-section within the E−0.5 limit (E corresponds to the initial kinetic energy of the electron). The yield of the TNI formed near 0.5 eV is further reduced due to the possibility of autodetachment nearly till the conical intersection at the C−Br distance of about 2.2 Å. This explains the significant intensity difference for the two resonances observed in the anion efficiency curves.
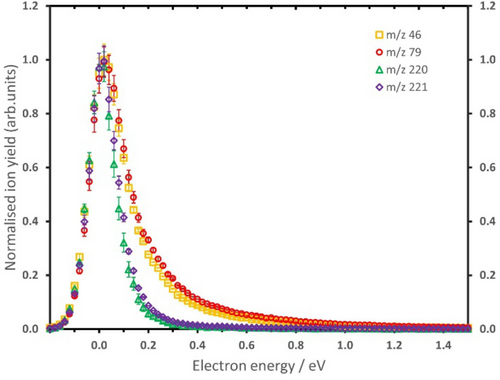
Anion efficiency curves of the fragment anions NO2− (m/z 46), Br− (m/z 79), (RRx−HNO2)− (m/z 220) and (RRx−NO2)− (m/z 221) formed upon electron attachment to RRx. The respective ion yields were scaled at the zero eV resonance. The statistical error margins shown for each data point refer to the standard error of the mean from individual measurements (see Supporting Information for more details). The corresponding symbols are as follows: m/z 46 (yellow squares), m/z 79 (red circles), m/z 220 (green triangles), and m/z 221 (indigo diamonds).
Conclusion
The present electron attachment results for RRx indicate that the compound is highly susceptible to low-energy electrons. The Br− represents the most abundant fragment anion just a few hundred microseconds after TNI formation. This observation disproves the hypothesis of a fast, simple dissociation process upon formation of the repulsive σ*(C−Br) state since the dissociation takes unexpectedly long for an exothermic dissociation process. Instead, the present calculations point out the formation of a non-covalent type of anionic complex Br−–(RRx−HNO2)⋅, which is found in the mass spectrum with high abundance at earlier times. The analogous mechanism applies to the reaction channel leading to the formation of NO2− which represents, on late timescales, an independent competitive pathway to the release of Br−. However, the feeding of the pathways on fast timescales occurs also upon a conical intersection of π*(NO2) and σ*(C−Br) states. Previously the σ* predissociation of π* resonance states was theoretically proposed to be an efficient DEA mechanism for various molecules.26-28 To mention a system of biological relevance, the formation of a single-strand break in (dry) DNA was proposed to be formed by the initial formation of a π* resonance of the nucleobase with subsequent electron transfer into a C−O σ* orbital of the phosphate group.29, 30 Experimental data also suggested the general preference of the π*→σ* coupling scheme since the non-dissociative π* resonances are characterized by longer lifetimes than σ* resonances with respect to spontaneous electron emission.26 For anionic RRx, the conical intersection, which is energetically found below the autodetachment continuum, allows to experimentally observe the reversed σ*→π* coupling scheme as well. Its appearance is indicated in the form of the resonance at ~0.5 eV in the NO2− ion yield. We also note that the involved π* state is of dissociative character here, unlike the molecular-non-dissociative π* resonances reported for other anionic systems.26-28
Radiobiological experiments indicated that RRx develops a high biological activity in cells. Experiments in the gas phase like the present one enable exploration of intermediate processes of radiation damage and to distinguish direct from indirect effects.31 A possible hypothesis may be that RRx may also become activated as a potential radiosensitizer by low-energy secondary electrons formed in irradiated cells. A previous electron attachment study with the radiosensitizer nimorazole demonstrated indeed such a possibility of activation mechanism.32 The present results for RRx indicate efficient release of nitrite, nitrogen dioxide, and nitrous acid, which can undergo a range of electron transfer, addition, and abstraction reactions,33 mainly resulting in a vasodilator effect34 or an increase of oxidative stress via enhanced OH formation.35 This would represent a novel mechanism for the oxygenation of hypoxic tumor cells in addition to the previously suggested reduction of serum nitrite to nitric oxide by RRx-bound hemoglobin.36 Reoxygenation of hypoxic tumor cells would lead to a lowered resistance of these cells towards ionizing radiation.37 However, it should be noted that the dissociation processes monitored presently on the microsecond timescale may be quenched in a molecular environment by, e.g., fast intermolecular energy dissipation38 or stabilizing proton transfer.30 In such a case, the anionic system relaxes before dissociation occurs. On the other hand, this may not apply to fast processes like shown by previous solution phase studies of an excited nucleoside anion.9 Thus the initially populated states of the TNI studied here will be the same in solution and therefore the present results provide deeper fundamental insight into the initial steps in the radiation chemistry of this potential radiosensitizer, irrespective of the environment. We also point out the importance of studying the dynamics of formation and degradation of negative ions for other classes of molecules, like for example photocatalysts used for photoredox-mediated organic reactions and polymerizations.39
Supporting Information
The Supporting Information presents the Experimental and Computational Methods, Supplementary Figures S1–S4, Supporting Information Table S1, Cartesian coordinates of structures, and raw data. Authors have cited additional references within the Supporting Information.40
Acknowledgments
This research was funded in part by the Austrian Science Fund (FWF) [Grant-DOIs 10.55776/P30332 and 10.55776/I5390]. For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. The computational results presented have been achieved using the HPC infrastructure LEO of the University of Innsbruck. The authors acknowledge the support of the Ministry of Education, Youth, and Sports of the Czech Republic via project CZ.02.01.01/00/22_008/0004649 QUEENTECH co-funded by the European Union. B.S. acknowledges the support by the Czech Science Foundation, Project No. 21-26601X. F.I. was supported by COST Action CA20129—Multiscale Irradiation and Chemistry Driven Processes and Related Technologies (MultIChem), supported by COST (European Cooperation in Science and Technology).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.