Skeletal Editing of Aromatic N-Heterocycles via Hydroborative Cleavage of C−N Bonds—Scope, Mechanism, and Property
Chunping Ren
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorDr. Bo Han
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorHui Guo
Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081 Beijing, P. R. China
Search for more papers by this authorWendi Yang
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorProf. Chungu Xia
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Xu-Hui Jin
Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Fang Wang
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Lipeng Wu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, 311121 Hangzhou, P. R. China
Search for more papers by this authorChunping Ren
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorDr. Bo Han
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorHui Guo
Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081 Beijing, P. R. China
Search for more papers by this authorWendi Yang
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
University of Chinese Academy of Sciences, 100049 Beijing, P. R. China
Search for more papers by this authorProf. Chungu Xia
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Xu-Hui Jin
Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081 Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Fang Wang
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Lipeng Wu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, 730000 Lanzhou, P. R. China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, 311121 Hangzhou, P. R. China
Search for more papers by this authorGraphical Abstract
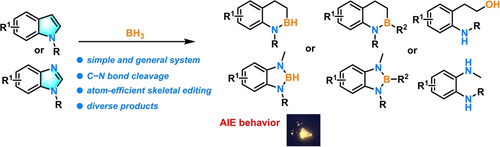
A straightforward and facile BH3-mediated skeletal editing of aromatic heterocycles via hydroborative cleavage of C−N bonds is reported. Structurally diverse products, including tetrahydrobenzo azaborinines, diazaboroles, O-anilinophenylethyl alcohols, benzene-1,2-diamines, and more were readily obtained. Density functional theory (DFT) calculations and natural bond orbital (NBO) analysis revealed an interesting and counterintuitive indole hydroboration phenomenon, including −BH2 shift from C3-position to C2-position. Moreover, the photophysical properties of the synthesized diazaboroles was studied, and an interestingly and pronounced aggregation-induced emission (AIE) behavior was disclosed.
Abstract
Skeletal editing of the core structure of heterocycles offers new opportunities for chemical construction and is a promising yet challenging research topic that has recently gained increasing interest. However, several limitations of the reported systems remain to be addressed. For example, the reagents employed are generally in high-energy, such as chlorocarbene precursors, nitrene species, and metal carbenes, which are also associated with low atomic efficiencies. Thus, the development of simple systems for the skeletal editing of heterocycles is still desired. Herein, a straightforward and facile BH3-mediated skeletal editing of readily available indoles, benzimidazoles, and several other aromatic heterocycles is reported. Structurally diverse products were readily obtained, including tetrahydrobenzo azaborinines, diazaboroles, O-anilinophenylethyl alcohols, benzene-1,2-diamines, and more. Density functional theory (DFT) calculations and natural bond orbital (NBO) analysis revealed a BH3-induced C−N bond cleavage reaction pathway. An exciting and counterintuitive indole hydroboration phenomenon of −BH2 shift from C3-position to C2-position was disclosed. Moreover, the photophysical properties of the synthesized diazaboroles were studied, and an interestingly and pronounced aggregation-induced emission (AIE) behavior was disclosed.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202407222-sup-0001-misc_information.pdf14.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. J. V. Julio Alvarez-Builla, José Barluenga, Modern Heterocyclic Chemistry, Vol. 4, Wiley, Weinheim, 2011;
10.1002/9783527637737 Google Scholar
- 1bR. D. Taylor, M. MacCoss, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845–5859.
- 2
- 2aA. M. Szpilman, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 9592–9628;
- 2bT. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal, S. W. Krska, Chem. Soc. Rev. 2016, 45, 546–576;
- 2cD. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson, A. Wood, Nat. Chem. 2018, 10, 383–394.
- 3
- 3aM. A. Larsen, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 4287–4299;
- 3bE. A. Meucci, S. N. Nguyen, N. M. Camasso, E. Chong, A. Ariafard, A. J. Canty, M. S. Sanford, J. Am. Chem. Soc. 2019, 141, 12872–12879;
- 3cJ. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk, Z. Shi, Nature 2019, 575, 336–340;
- 3dL. Guillemard, N. Kaplaneris, L. Ackermann, M. J. Johansson, Nat. Chem. Rev. 2021, 5, 522–545;
- 3eB. Zhao, B. Prabagar, Z. Shi, Chem 2021, 7, 2585–2634.
- 4
- 4aJ. Jurczyk, M. C. Lux, D. Adpressa, S. F. Kim, Y.-h. Lam, C. S. Yeung, R. Sarpong, Science 2021, 373, 1004–1012;
- 4bJ. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong, M. D. Levin, Nat. Synth. 2022, 1, 352–364;
- 4cF. Liu, L. Anand, M. Szostak, Chem. Eur. J. 2023, 29, e202300096.
- 5
- 5aA. Sattler, G. Parkin, Nature 2010, 463, 523–526;
- 5bS. Hu, T. Shima, Z. Hou, Nature 2014, 512, 413–415;
- 5cS. Hu, G. Luo, T. Shima, Y. Luo, Z. Hou, Nat. Commun. 2017, 8, 1866;
- 5dX. Qiu, Y. Sang, H. Wu, X.-S. Xue, Z. Yan, Y. Wang, Z. Cheng, X. Wang, H. Tan, S. Song, G. Zhang, X. Zhang, K. N. Houk, N. Jiao, Nature 2021, 597, 64–69.
- 6
- 6aG. L. Ciamician, M. Dennstedt, Ber. Dtsch. Chem. Ges. 1881, 14, 1153–1163;
10.1002/cber.188101401240 Google Scholar
- 6bT. Tsuchiya, S. Okajima, M. Enkaku, J. Kurita, J. Chem. Soc. Chem. Commun. 1981, 211–213;
- 6cD. L. Boger, Chem. Rev. 1986, 86, 781–793;
- 6dZ. Chen, M. L. Trudell, Chem. Rev. 1996, 96, 1179–1194;
- 6eK. Maeda, T. Hayashi, Bull. Chem. Soc. Jpn. 2006, 44, 533–536;
- 6fS. Suzuki, Y. Segawa, K. Itami, J. Yamaguchi, Nat. Chem. 2015, 7, 227–233;
- 6gJ. Wang, H. Lu, Y. He, C. Jing, H. Wei, J. Am. Chem. Soc. 2022, 144, 22433–22439;
- 6hB. W. Joynson, L. T. Ball, Helv. Chim. Acta. 2023, 106, e202200182;
- 6iZ. Liu, P. Sivaguru, Y. Ning, Y. Wu, X. Bi, Chem. Eur. J. 2023, 29, e202301227.
- 7
- 7aB. Chattopadhyay, V. Gevorgyan, Angew. Chem. Int. Ed. 2012, 51, 862–872;
- 7bP. Anbarasan, D. Yadagiri, S. Rajasekar, Synthesis 2014, 46, 3004–3023;
- 7cT. Miura, J. Synth. Org. Chem. Jpn. 2015, 73, 1200–1211.
- 8
- 8aM. Bhanuchandra, K. Murakami, D. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 10234–10238;
- 8bD. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 7162–7166;
- 8cH. Yorimitsu, D. Vasu, M. Bhanuchandra, K. Murakami, A. Osuka, Synlett 2016, 27, 1765–1774;
- 8dM. Bhanuchandra, H. Yorimitsu, A. Osuka, Org. Lett. 2016, 18, 384–387;
- 8eH. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, 15315–15318;
- 8fM. Onoda, Y. Koyanagi, H. Saito, M. Bhanuchandra, Y. Matano, H. Yorimitsu, Asian J. Org. Chem. 2017, 6, 257–261;
- 8gY. Kurata, S. Otsuka, N. Fukui, K. Nogi, H. Yorimitsu, A. Osuka, Org. Lett. 2017, 19, 1274–1277;
- 8hK. Nogi, H. Yorimitsu, Chem. Commun. 2017, 53, 4055–4065;
- 8iP. Xu, E.-U. Würthwein, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2017, 56, 13872–13875;
- 8jS. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 3855–3860;
- 8kH. Saito, H. Yorimitsu, Chem. Lett. 2019, 48, 1019–1028.
- 9B. D. Dherange, P. Q. Kelly, J. P. Liles, M. S. Sigman, M. D. Levin, J. Am. Chem. Soc. 2021, 143, 11337–11344.
- 10J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein, B. Morandi, Science 2022, 377, 1104–1109.
- 11E. E. Hyland, P. Q. Kelly, A. M. McKillop, B. D. Dherange, M. D. Levin, J. Am. Chem. Soc. 2022, 144, 19258–19264.
- 12J. Woo, A. H. Christian, S. A. Burgess, Y. Jiang, U. F. Mansoor, M. D. Levin, Science 2022, 376, 527–532.
- 13G. L. Bartholomew, F. Carpaneto, R. Sarpong, J. Am. Chem. Soc. 2022, 144, 22309–22315.
- 14A nickel-catalyzed boron insertion into alkyl ether bonds was reported: H. Lyu, I. Kevlishvili, X. Yu, P. Liu, G. Dong, Science 2021, 372, 175–182.
- 15A. Jayaraman, H. Powell-Davies, F.-G. Fontaine, Tetrahedron 2019, 75, 2118–2127.
- 16
- 16aL. Weber, L. Böhling, Coord. Chem. Rev. 2015, 284, 236–275;
- 16bK. E. Krantz, S. L. Weisflog, N. C. Frey, W. Yang, D. A. Dickie, C. E. Webster, R. J. Gilliard, Jr., Chem. Eur. J. 2020, 26, 10072–10082;
- 16cC.-W. Ju, B. Li, L. Li, W. Yan, C. Cui, X. Ma, D. Zhao, J. Am. Chem. Soc. 2021, 143, 5903–5916.
- 17
- 17aT. P. Lin, J. C. Peters, J. Am. Chem. Soc. 2013, 135, 15310–15313;
- 17bT. P. Lin, J. C. Peters, J. Am. Chem. Soc. 2014, 136, 13672–13683;
- 17cH. Schubert, W. Leis, H. A. Mayer, L. Wesemann, Chem. Commun. 2014, 50, 2738–2740;
- 17dK. D. Spielvogel, G. Durgaprasad, S. R. Daly, Organometallics 2022, 41, 1344–1352.
- 18Deposition number 2250387 (for compound 5 n) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 19H. Talukdar, D. Gogoi, P. Phukan, Tetrahedron 2023, 132, 133251.
- 20
- 20aJ. Legare Lavergne, A. Jayaraman, L. C. Misal Castro, E. Rochette, F. G. Fontaine, J. Am. Chem. Soc. 2017, 139, 14714–14723;
- 20bA. Jayaraman, L. C. Misal Castro, V. Desrosiers, F.-G. Fontaine, Chem. Sci. 2018, 9, 5057–5063;
- 20cS. Rej, N. Chatani, Angew. Chem. Int. Ed. 2022, 61, e202209539.