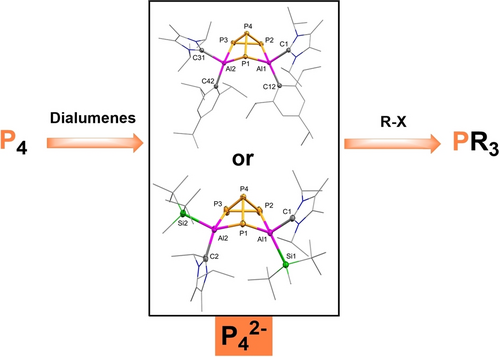
Dialumene-Mediated Production of Phosphines through P4 Reduction
Graphical Abstract
The formation of phosphorus-rich alanes featuring butterfly-like geometries is achieved. The two-electron reduction products feature a unique P42- structure and are able to be the source of P3-. It is that the treatment of different electrophiles to them under mild conditions resulted different phosphines, which avoids the requirements of high temperature and/or high pressure for the preparation of the useful phosphines in industry.
Abstract
The formation of phosphorus-rich alanes featuring butterfly-like geometries is achieved. The two-electron reduction products feature a unique P42− structure and can act as a source of P3−. The treatment of these phosphorus containing products with electrophiles under mild conditions results in the formation of different phosphines. This approach eliminates the need for high temperatures and/or high pressures, which are commonly required in industrial processes for the preparation of useful phosphines.The activation and further functionalization of white phosphorus (P4) by main group complexes has become an increasingly studied topic in recent times. Herein, we report the controlled formation of phosphorus-rich alanes featuring butterfly-like geometries from the selective reaction of P4 with dialumenes, ([L(IiPr)Al]2) (1: L=Tripp=2,4,6-iPr3C6H2; 2: L=tBu2MeSi; IiPr=[MeCN(iPr)]2C)). The two-electron-reduction product of P4 features a P42− structure and is shown to be able to act as a source of P3−. Treatments of different electrophiles (e.g., chlorotrimethylsilane (Me3SiCl), iodotrimethylsilane (Me3SiI), HCl, or acetyl chloride (CH3COCl)) with these alanes under mild conditions gave the corresponding phosphines (e.g., P(SiMe3)3, PH3, or P(COCH3)3).
The chemistry of multiple bonded p-block complexes has been the subject of intense research since the seminal work of West and Yoshifuji, reporting the first heavy main group multiple bonds (Si=Si and P=P, respectively) in 1981.1 The weaker π-bond of the heavier p-block elements renders such species highly reactive. Harnessing this reactivity can lead to “transition metal-like” reactivity, such as the activation/functionalization of relatively inert substrates and catalysis.2 This reactivity is of particular interest when observed for highly abundant, low-toxicity elements such as silicon and aluminum, as they present potential alternatives to generally more scarce and toxic transition metal systems which are used heavily in industry. As a result, both the search for new multiple bonded main group complexes and subsequent investigations into their reactivity presents a fruitful avenue to new abundant element-mediated synthetic approaches.
The industrial production of useful monophosphorus (P1) products typically relies on the use of white phosphorus (P4) as a chemical feedstock, which is then oxidised by elemental chlorine to give PCl3 and PCl5 (alongside many other by-products) and subsequent functionalization.3 To mitigate the use of hazardous chlorine gas and improve the atom economy of such chemistry, the direct functionalization of P4 to phosphorus-based products is highly desirable.4 As such, the stoichiometric reactivity of both main group5 and transition metal-based organometallic complexes6 towards P4 has been well-explored. While P−P bond cleavage and the formation of new element-phosphorus compounds are now well known for many elements of the periodic table, the subsequent release of P1 products is far rarer.4
The controlled activation of P4 by main group compounds is most commonly observed for those in a low oxidation state, as they possess an amphiphilic nature (an empty p orbital and a lone pair of electrons). For example, both neutral and anionic aluminum(I) reagents have been shown to react with P4 yielding various formal reduction products such as the P4 insertion isomers VII7 and IX8 (Figure 1) and the P4 fragmentation compounds VIII9 and X.10 Meanwhile, the reactivity of unsaturated main group complexes towards white phosphorus is limited to four examples: that of two disilenes (I and IV, Figure 1),11 a diboraallene (III)12, and a phosphasilenyl-tetrylene reported recently by our group.13
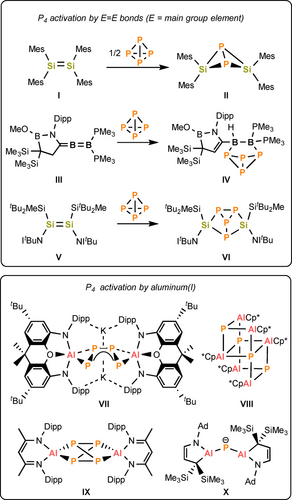
The selected examples of P4 activation by π-bonded main group complexes and the known products of P4 activation as mediated by Al(I) reagents (Dipp=2,6-iPr2−C6H3, Mes=2,4,6-Me3−C6H2, ItBuN=[HCN(tBu)]2C=N).
Since our 2017 report of the first dialumene (Al=Al),14 we and others have been exploring the reactivity of this class of compound towards various substrates such as alkenes, alkynes, CO2, and H2.15 In this contribution, we disclose our efforts to extend the dialumene reactivity to white phosphorus. We demonstrate that both aryl- and silyl-substituted dialumenes selectively afford novel P4 activation products which are amenable to further functionalization on exposure to electrophiles, affording useful phosphines.
Aryl/silyl stabilized dialumenes 1 ([Tripp(IiPr)Al]2) and 2 ([SitBu2Me(IiPr)Al]2) were synthesized in good yields following the reported procedures (Tripp=2,4,6-iPr3-C6H2; IiPr={MeCN(iPr)}2C).14, 15 Treatment of 1 with one equivalent of white phosphorus in toluene at room temperature gave a brown solid after workup (Figure 2). Over time it was apparent via NMR spectroscopic monitoring that this initial product was converting into a new compound, and it was possible to grow crystals of the product. SC-XRD data16 confirms this compound to be [Tripp(IiPr)Al]2P4 (3-cis, Figure 2), indicating that one molecule of P4 has inserted into the Al=Al double bond of 1. It features a butterfly-like geometry with adjacent IiPr and Tripp ligands on the Al centers positioned in a cis manner. To the best of our knowledge, this is a new mode of P4 activation with the aluminum centers. This reactivity is analogous to the recently reported insertion of P4 into a Si=Si double bond (V and VI, Figure 1).11c
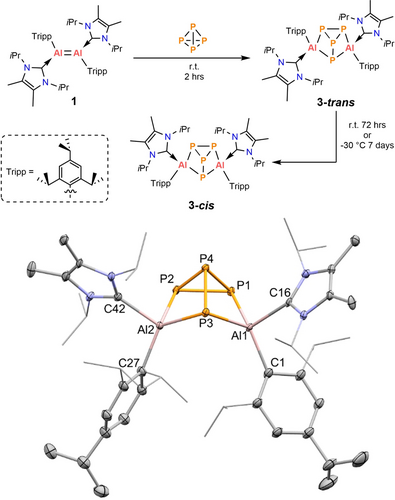
Reaction of P4 with dialumene (1) and the molecular structure of 3-cis, ellipsoids are set at the 50 % probability level; hydrogen atoms and co-crystallized solvent molecules are omitted for clarity. Selected bond lengths (Å) and angles (°): Al1−P1 2.400(2), Al1−P3 2.349(2), Al1−C1 2.020(3), Al1−C16 2.100(6), Al2−P2 2.376(2), Al2−P3 2.335(2), Al2−C27 1.992(4), Al2−C42 2.095(6), P1−P2 2.197(2), P1−P4 2.242(2), P2−P4 2.220(2), P3−P4 2.298(2), P1−Al1−P3 93.84(8), P2−Al2−P3 94.04(8), P2−P1−Al1 106.90(8), P4−P1−Al1 79.34(7), Al1−P3−Al2 100.59(8).
The P−P bond lengths (av. 2.239 Å) vary in the range from 2.197(2) Å to 2.298(2) Å, which fall in the range of typical P−P single bond (cf. 2.1891(7)-2.2937(7) Å11c in the P4 insertion product of Si=Si VI, or 2.209(5) Å5b in P4), and are slightly longer than the observed in a formal [P4]4− obtained from the reduction by Al(I), IX (av. 2.29 Å).8 But they are longer than the bonds in VII (ca. 2.13 Å) due to the presence of a delocalized π-bond within the [P4]4− unit.7 The Al−P bond lengths (av. 2.365 Å) of 3-cis are in the range of those in the P4Al6 framework of VIII (2.31–2.42 Å),9 but shorter than those of IX (av. 2.37 Å)11c and VII (av. 2.54 Å).7 Additionally, they are longer than that in the P4 fragmentation product from the alumanyl anion, X (2.246(1) Å) bearing highly polarized Alδ+-Pδ− bonds.10
The 31P{1H} NMR spectrum of 3-cis showed three signals at −55.1 ppm (d, P3), −138.3 ppm (d, P1 and P2) and −159.4 ppm (td, P4), which are shifted downfield compared to that observed for VI (−44.0 ppm (dd), 106.6 ppm (dt), −217.6 ppm (dt))11c and X (−295.4 ppm).10 They appeared at higher chemical shifts than that of IX (78.6 ppm)8 and VII (374.2 ppm (br), 54.3 ppm (br)).7 Compound 3-cis is highly thermostable without any change in C6D6 when heated to 80 °C for 72 hours.
We next wanted to elucidate the identity of the initial product formed from the reaction of 1 and P4 (Figure 2). Based on the geometry of the starting material 1, in which the Tripp and NHC moieties are arranged in a trans configuration about the Al=Al bond, we propose that this geometry is obtained upon initial reaction with P4 giving 3-trans. The 31P{1H} NMR data showed four signals at −52.7 ppm (d, P3), −128.8 ppm (dd, P1), −138.7 ppm (dd, P2), and −147.8 (ddd, P4), which shifted slightly downfield than the observed in 3-cis. P1 and P2 featuring quartet peaks at different shifts indicating they are in asymmetric chemical environments. It was not possible to isolate X-ray quality crystals of 3-trans, so to provide evidence of its formation, we calculated the 31P{1H} NMR chemical shifts and coupling constants and compared them to the results of the experimental 31P{1H} NMR spectrum. The simulated spectrum of the proposed 3-trans reproduces the experimentally observed pattern (Figure S61 in the supporting information). Quantum chemical calculations on the proposed mechanism for the reaction of compound 1 with P4 giving 3-trans and 3-cis are presented in Figure S58 in the supporting information. We propose that the formation of 3-cis takes place via the initial formation of 3-trans that was observed by multinuclear NMR spectroscopy, which then isomerized to the energetically more favoured isomer 3-cis. The overall process is exergonic by 84.5 kcal mol−1 relative to 1+P4. Relaxed surface scans show that the NHC dissociation from Al1 in 3-trans and 3-cis, is barrierless, and results in the essentially similar intermediates 3′ and 3′′ (Figure 3), which only differ from each other by the relative orientation of the aryl substituent at Al1. Thus, we propose that the 3-trans to 3-cis isomerization proceeds via the NHC dissociation from 3-trans to form 3′, which is only endergonic by 14.6 kcal mol−1. 3′ isomerizes to 3′′ at 13.9 kcal mol−1, followed by re-association of the NHC at Al1.
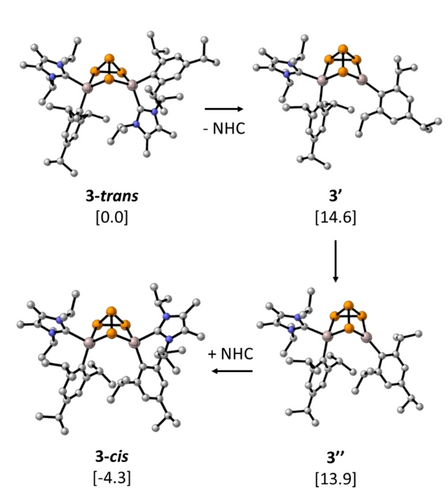
Proposed mechanism for 3-trans→3-cis isomerization. Optimized geometries are presented. Hydrogen atoms are omitted for clarity. The free energies (kcal mol−1) relative to 3-trans are shown in square brackets.
3-cis is calculated to be energetically more favourable by 4.3 kcal mol−1, therefore although the low NHC dissociation energies are expected to allow 3-trans and 3-cis to exist in thermodynamic equilibrium under ambient conditions, only 3-cis can be observed when the equilibrium is reached. In summary, these results indicate that the reaction of 1 and P4 first goes via a trans conformation 3-trans which slowly isomerises to give the thermodynamic product 3-cis at −30 °C after 7 days or at room temperature after 72 hours (Figure 2).
We were also interested to see whether the silyl substituted dialumene ([SitBu2Me(IiPr)Al]2 2) would react differently towards P4. Treatment of one equivalent of white phosphorus with 2 in benzene at room temperature after 2 hours yielded a pale orange powder after work up (Figure 4). Recrystallisation of the orange powder and subsequent SC-XRD analysis shows the solid state structure to be [SitBu2Me(IiPr)Al]2P4 4, which possesses an analogous Al2P4 cage motif to 3-cis, but the NHC and silyl ligands are in a trans configuration (Figure 4). The distances between the Al centers and NHC (Al1−C1 2.066(3) Å, Al2−C12 2.103(3) Å) as well as P−P bond lengths (av. 2.237 Å) in Figure 4 were comparable with those of 3-cis (A1−C16 2.100(6) Å, Al2−C42 2.095(6) Å, P−P av. 2.239 Å). The angles of P−Al−P in 4 (av. 93.33°) are also similar to 3-cis (av. 93.44°). The 31P{1H} NMR spectrum shows a multiplet for P4 at −118.5 ppm which is significantly downfield shifted compared with the signal of P4 in 3-trans (−147.8 ppm) and 3-cis (−159.4 ppm).
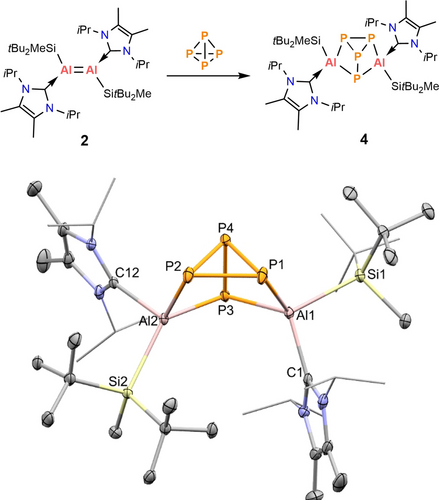
Reaction of P4 and dialumene (2). Molecular structure of 4 in the solid state. Ellipsoids are set at the 50 % probability level; hydrogen atoms and co-crystallized solvent molecules are omitted for clarity. Selected bond lengths (Å) and angles (°): Al1−P1 2.3913(12), Al1−P3 2.3426(11), Al1−C1 2.066(3), Al1−Si1 2.4997(12), Al2−P2 2.4013(11), Al2−P3 2.3657(11), Al2−C12 2.103(3), Al2−Si2 2.5198(11), P1−P2 2.1978(11), P1−P4 2.2325(12), P2−P4 2.2263(13), P3−P4 2.2907(11), P1−Al1−P3 93.83(4), Al1−P3−Al2 105.08(4), P2−Al2−P3 92.83(4).
Contrary to 3-trans, it appears that 4 with trans configuration does not convert into the cis isomer on standing in solution nor on heating. The variable temperature 31P{1H} NMR experiments indicated a minor shift of the signals on heating, including a coalescing of the signal for P2/P4 but on cooling it reverts to the original shifts (Figure S1 and S36 in the supporting information). Quantum chemical calculations show that the formation of 4 from the reaction of 2 and P4 is exergonic by 73.8 kcal mol−1. The trans isomer of 4 is more favoured by 4.1 kcal mol−1 compared to the cis isomer. When 4 was heated to 80 °C for 2 hours, an intractable mixture of products was observed in the 1H and 31P{1H} NMR spectra (Figure S33 in the supporting information).
Considering the highly reduced and unsaturated nature of the P42− unit in 3-trans/cis and 4, we were curious as to whether further functionalization was possible. Thus, reactions with the electrophiles Me3SiCl, Me3SiI, HCl (4 M in dioxane) and CH3COCl were conducted (Figure 5-6, and Table S1-3 in the supporting information). Treating 3-trans with an excess of Me3SiX (X=Cl, I) in C6D6 at 80 °C gave aluminum dihalides Tripp(IiPr)AlX2 5 and P(SiMe3)3, which was confirmed by in situ 1H and 31P{1H} NMR spectroscopy. PH3 and P(OCCH3)3 were formed from the reactions of 3-trans with 4 M HCl in dioxane and CH3COCl, respectively, at ambient temperature. When 3-cis was used, P(SiMe3)3 was formed with high P conversion rate under milder conditions (Table S2 in the supporting information), as well as PH3 or P(OCCH3)3 with a comparable P conversion rate.

Reactivity of 3-trans with various electrophiles. [a] Yield calculated by (The ratio of phosphine vs. 1 eqv. PPh3)/4.
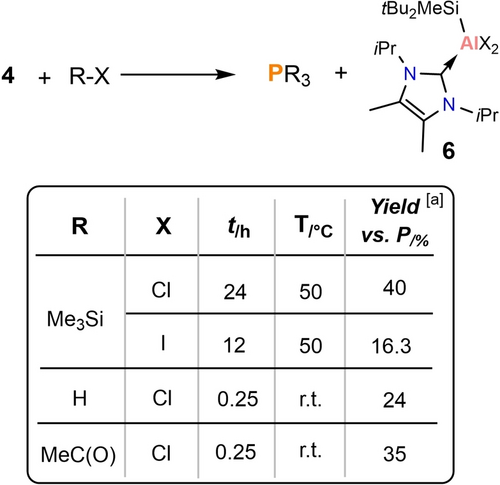
Reactivity of 4 with various electrophiles. [a] Yield calculated by (The ratio of phosphine vs. 1 eqv. PPh3)/4.
The reaction of 4 with Me3SiX (X=Cl or I) gives comparable conversion rates to 3-trans at lower temperatures (Figure 6). The results were tracked by in situ 1H and 31P{1H} NMR spectroscopy, showing that P(SiMe3)3 and [SitBu2Me(NHC)AlX2 (6) were formed. Compound 4 reacted with HCl (4 M in dioxane), and CH3COCl at room temperature forming PH3, and P(OCCH3)3, respectively. These results demonstrate that the P4 activation products by dialumenes can release P1 compounds on reaction with electrophiles under mild conditions. Additionally, the by-product LAlX2 is the precursor to the dialumenes14, 15 allowing for the potential regeneration of the starting material.
In conclusion, we have utilised dialumenes ([L(IiPr)Al]2, L=Tripp (1), or SitBu2Me (2)) in the activation and subsequent functionalisation of white phosphorus (P4). Their reactivity towards white phosphorus resulted in the reduction of P4 to P42− across the Al=Al double bond. Depending on the dialumene employed, we were successful in isolating the P-rich alanes in cis or trans configurations. This is the first report to elucidate the mechanism of the reaction of P4 with a π-bond resulting in cis configuration from trans, supported with DFT calculations. In addition, subsequent reactivity studies with electrophiles demonstrated that both [Tripp(IiPr)Al]2P4 3-trans/cis and [SitBu2Me(IiPr)Al]2P4 4 acted as a P3− source, giving access to various useful phosphines. The mechanism of the formation of AlP4Al core is currently under investigation. This is another step forward in the field of main group aided P4 functionalisation and further reactivity studies of 3-trans/cis and 4 are currently ongoing.
Acknowledgments
We are grateful to the European Research Council (ALLOWE 101001591) for financial support. H.X. gratefully acknowledges financial support from the China Scholarship Council (CSC). M.M.D.R and S.F. thank the Humboldt Foundation for financial support. We thank Florian Tschernuth for VT NMR and Ivan Antsiburov for LIFDI Mass. The authors gratefully acknowledge the Leibniz Supercomputing Centre for funding this project by providing computing time on its Linux-Cluster. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.