Beyond Tertiary Amines: Introducing Secondary Amines by Palladium/Norbornene-Catalyzed Ortho Amination
Xin Liu
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorQi Zhu
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorCorresponding Author
Guangbin Dong
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorXin Liu
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorQi Zhu
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorCorresponding Author
Guangbin Dong
Department of Chemistry, University of Chicago, Chicago, Illinois, 60637 United States
Search for more papers by this authorGraphical Abstract
Abstract
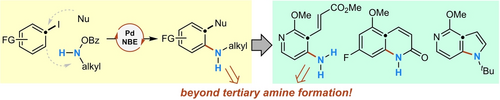
Since the discovery of the palladium/norbornene (Pd/NBE)-catalyzed ortho amination in 2013, escaping the limitation of only yielding tertiary anilines has been a long-standing challenge. Here, we describe that, by carefully choosing the phosphine ligand and NBE mediator, the installation of a N-mono-alkylamino group becomes feasible. The reaction tolerates a wide range of aryl iodide substrates and various N-mono-tertiary alkylamine-derived electrophiles. Both ipso alkenylation and alkynylation can be realized. The synthetic utility of this method is exemplified by the formation of primary amino group via selective deprotection and streamlined access to N-heterocycles. Preliminary success of installing a bulky N-secondary alkylamino group and a mechanistic understanding of the decomposition pathways of mono N-alkylamine electrophiles have been obtained.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202404042-sup-0001-misc_information.pdf4.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Ricci, Amino Group Chemistry: from Synthesis to the Life Sciences, John Wiley & Sons 2008;
- 1bN. A. McGrath, M. Brichacek, J. T. Njardarson, J. Chem. Educ. 2010, 87, 1348–1349.
- 2S. Rohrbach, A. J. Smith, J. H. Pang, D. L. Poole, T. Tuttle, S. Chiba, J. A. Murphy, Angew. Chem. Int. Ed. 2019, 58, 16368–16388.
- 3
- 3aJ. F. Hartwig, E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley-Interscience, New York 2002;
- 3bL. Jiang, S. L. Buchwald, Metal-Catalyzed Cross-Coupling Reactions, 2nd, completely rev. and enl. ed., Wiley-VCH, Weinheim 2004;
- 3cJ. F. Hartwig, Acc. Chem. Res. 2008, 41, 1534–1544;
- 3dY. Aubin, C. Fischmeister, C. M. Thomas, J. L. Renaud, Chem. Soc. Rev. 2010, 39, 4130–4145;
- 3eD. Maiti, B. P. Fors, J. L. Henderson, Y. Nakamura, S. L. Buchwald, Chem. Sci. 2011, 2, 2428–2428;
- 3fD. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27–50.
- 4
- 4aF. Monnier, M. Taillefer, Amination and Formation of Sp2C-N Bonds 2013, 46, 173–204;
- 4bC. A. Echeverry-Gonzalez, V. V. Kouznetsov, in Copper in N-Heterocyclic Chemistry, Elsevier 2021, pp. 399–430.
10.1016/B978-0-12-821263-9.00011-4 Google Scholar
- 5
- 5aD. M. T. Chan, K. L. Monaco, R. P. Wang, M. P. Winters, Tetrahedron Lett. 1998, 39, 2933–2936;
- 5bD. A. Evans, J. L. Katz, T. R. West, Tetrahedron Lett. 1998, 39, 2937–2940;
- 5cP. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan, A. Combs, Tetrahedron Lett. 1998, 39, 2941–2944.
- 6
- 6aA. M. Berman, J. S. Johnson, J. Am. Chem. Soc. 2004, 126, 5680–5681;
- 6bC. E. Hendrick, Q. Wang, J. Org. Chem. 2017, 82, 839–847.
- 7
- 7aM. L. Louillat, F. W. Patureau, Chem. Soc. Rev. 2014, 43, 901–910;
- 7bY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301.
- 8For selected reviews, see:
- 8aJ. Jiao, K. Murakami, K. Itami, ACS Catal. 2016, 6, 610–633;
- 8bH. Kim, S. Chang, ACS Catal. 2016, 6, 2341–2351.
- 9
- 9aZ. Dong, G. Dong, J. Am. Chem. Soc. 2013, 135, 18350–18353;
- 9bZ. Dong, G. Lu, J. C. Wang, P. Liu, G. Dong, J. Am. Chem. Soc. 2018, 140, 8551–8562.
- 10For the seminal work, see:
- 10aM. Catellani, F. Frignani, A. Rangoni, Angew. Chem. Int. Ed. 1997, 36, 119–122; For selected reviews, see:
- 10bJ. T. Ye, M. Lautens, Nat. Chem. 2015, 7, 863–870;
- 10cN. Della Ca, M. Fontana, E. Motti, M. Catellani, Acc. Chem. Res. 2016, 49, 1389–1400;
- 10dJ. C. Wang, G. Dong, Chem. Rev. 2019, 119, 7478–7528;
- 10eA. D. Marchese, B. Mirabi, M. Lautens, Synthesis 2023, 55, 2285–2303.
- 11For a recent review, see: X. Dong, Q. Liu, Y. H. Dong, H. Liu, Chem. Eur. J. 2017, 23, 2481–2511.
- 12For selected examples of ortho amination, see:
- 12aZ. Y. Chen, C. Q. Ye, H. Zhu, X. P. Zeng, J. J. Yuan, Chem. Eur. J. 2014, 20, 4237–4241;
- 12bC. Q. Ye, H. Zhu, Z. Y. Chen, J. Org. Chem. 2014, 79, 8900–8905;
- 12cP. X. Zhou, Y. Y. Ye, J. W. Ma, L. Zheng, Q. Tang, Y. F. Qiu, B. Song, Z. H. Qiu, P. F. Xu, Y. M. Liang, J. Org. Chem. 2014, 79, 6627–6633;
- 12dS. F. Pan, X. Ma, D. N. Zhong, W. Z. Chen, M. C. Liu, H. Y. Wu, Adv. Synth. Catal. 2015, 357, 3052–3056;
- 12eH. Shi, D. J. Babinski, T. Ritter, J. Am. Chem. Soc. 2015, 137, 3775–3778;
- 12fF. G. Sun, Z. H. Gu, Org. Lett. 2015, 17, 2222–2225;
- 12gB. Luo, J. M. Gao, M. Lautens, Org. Lett. 2016, 18, 4166–4169;
- 12hJ. Wang, Z. H. Gu, Adv. Synth. Catal. 2016, 358, 2990–2995;
- 12iL. X. Fan, J. J. Liu, L. Bai, Y. Y. Wang, X. J. Luan, Angew. Chem. Int. Ed. 2017, 56, 14257–14261;
- 12jW. C. Fu, B. Zheng, Q. Y. Zhao, W. T. K. Chan, F. Y. Kwong, Org. Lett. 2017, 19, 4335–4338;
- 12kA. Whyte, M. E. Olson, M. Lautens, Org. Lett. 2018, 20, 345–348;
- 12lB. S. Zhang, Y. K. Li, Y. An, Z. Zhang, C. Liu, X. G. Wang, Y. M. Liang, ACS Catal. 2018, 8, 11827–11833;
- 12mQ. W. Gao, Z. S. Liu, Y. Hua, L. S. Li, H. G. Cheng, H. J. Cong, Q. H. Zhou, Chem. Commun. 2019, 55, 8816–8819;
- 12nY. An, B. S. Zhang, Z. Zhang, C. Liu, X. Y. Gou, Y. N. Ding, Y. M. Liang, Chem. Commun. 2020, 56, 5933–5936;
- 12oB. S. Zhang, Y. K. Li, X. Y. Gou, Z. Zhang, Y. An, X. G. Wang, Y. M. Liang, Chem. Commun. 2020, 56, 9202–9205;
- 12pZ. Zhang, B. S. Zhang, K. L. Li, Y. An, C. Liu, X. Y. Gou, Y. M. Liang, J. Org. Chem. 2020, 85, 7817–7839;
- 12qC. T. Wang, M. Li, Y. N. Ding, W. X. Wei, Z. Zhang, X. Y. Gou, R. Q. Jiao, Y. T. Wen, Y. M. Liang, Org. Lett. 2021, 23, 786–791;
- 12rZ. C. Bao, C. Q. Wu, J. B. Wang, Eur. J. Org. Chem. 2023, 26;
- 12sX. Y. Du, X. Z. Yang, H. Wang, X. G. Li, M. R. Wang, X. Li, Y. Tao, Y. X. Yang, X. Q. Tan, F. Ren, P. X. Zhou, Y. M. Liang, Org. Chem. Front. 2023, 10, 898–904;
- 12tB. S. Zhang, S. Y. Zhao, S. X. Li, W. Y. Jia, Y. X. Yang, Y. M. Wang, X. Y. Gou, Y. M. Liang, X. C. Wang, Z. J. Quan, J. Org. Chem. 2023, 88, 1786–1795.
- 13
- 13aJ. Li, Y. Yang, Y. X. Liu, Q. Liu, L. Z. Zhang, X. J. Li, Y. H. Dong, H. Liu, Org. Lett. 2021, 23, 2988–2993;
- 13bA. J. Rago, G. Dong, Org. Lett. 2021, 23, 3755–3760.
- 14X. Liu, J. Wang, G. Dong, J. Am. Chem. Soc. 2021, 143, 9991–10004.
- 15Z. Wu, X. L. Xu, J. Wang, G. Dong, Science 2021, 374, 734–740.
- 16
- 16aF. F. Wu, H. H. Wang, W. Z. Chen, Appl. Organomet. Chem. 2019, 33;
- 16bM. Ghasemi, F. Jafarpour, A. Habibi, Synthesis 2020, 52, 2092–2098;
- 16cF. Jafarpour, N. Jalalimanesh, M. Teimouri, M. Shamsianpour, Chem. Commun. 2015, 51, 225–228.
- 17H. Noda, Y. Asada, M. Shibasaki, Org. Lett. 2020, 22, 8769–8773.
- 18S. Lin, B. Lin, Z. T. Zhang, J. H. Chen, Y. S. Luo, Y. Z. Xia, Org. Lett. 2022, 24, 3302–3306.
- 19P. X. Shen, X. C. Wang, P. Wang, R. Y. Zhu, J. Q. Yu, J. Am. Chem. Soc. 2015, 137, 11574–11577.
- 20R. Li, G. Dong, J. Am. Chem. Soc. 2020, 142, 17859–17875.
- 21M. Lautens, S. Piguel, Angew. Chem. Int. Ed. 2000, 39, 1045–1046.
10.1002/(SICI)1521-3773(20000317)39:6<1045::AID-ANIE1045>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 22R. Li, G. Dong, Angew. Chem. Int. Ed. 2018, 57, 1697–1701.
- 23J. Wang, Z. Dong, C. Yang, G. Dong, Nat. Chem. 2019, 11, 1106–1112.
- 24Attempts to use aryl bromides were unfruitful at this stage, leading to mainly recovery of the substrate.
- 25Ph-Davephos is a pre-ligand in this system, which is converted to a phosphafluorene ligand. For our recent study, see: J. Wang, Y. Zhou, X. Xu, P. Liu, G. Dong, J. Am. Chem. Soc. 2020, 142, 3050–3059. Ph-Davephos gave lower yield than L1 for model substrate 1 a (58 %, NMR yield) and was far less efficient for electron-deficient substrates.
- 26Other ipso termination, such as hydrogen or Suzuki quenches, remains challenging at this stage, leading to ortho, ipso diamination products, see Scheme 3 .
- 27
- 27aE. Motti, M. Rossetti, G. Bocelli, M. Catellani, J. Organomet. Chem. 2004, 689, 3741–3749;
- 27bC. H. Lei, X. J. Jin, J. R. Zhou, ACS Catal. 2016, 6, 1635–1639;
- 27cW. W. Lv, Y. H. Chen, S. Wen, D. Ba, G. L. Cheng, J. Am. Chem. Soc. 2020, 142, 14864–14870.
- 28J. Ye, M. Lautens, in Remote C-H Bond Functionalizations: Methods and Strategies in Organic Synthesis 2021, pp. 56–114.
10.1002/9783527824137.ch3 Google Scholar
- 29To further check this result, the use of a heavier amine was attempted under the same conditions, which also did not yield any ipso amination product.
- 30Efforts to trap the nitrene species by adding different alkenes were unfruitful at this stage.
- 31Deposition number 2303994 (for 10), 2308602 (for 4 as) and 2303993 (for 11) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 32H. Shimizu, Y. Kawano, S. Ishikawa, Y. Uematsu, T. Shinohara, M. Itotani, Y. Haraguchi, I. Takemura, A. Kaneshige, Y. Nakai, N. Hariguchi, Y. Hayashi, M. Matsumoto, (Otsuka Pharmaceutical Co., Ltd.), WO 2016, p. 443 pages.
- 33S. Das, Asian J. Org. Chem. 2023, 12, e202300267.