Perfluorooxosulfate Salts as SOF4-Gas-Free Precursors to Multidimensional SuFEx Electrophiles
Armir Zogu
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorKarim Ullah
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Department of Chemistry and Technologies of Drug, Sapienza, University of Rome, P.le A. Moro 5, 00185 Rome, Italy
Search for more papers by this authorStefanos Spanopoulos
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Ermal Ismalaj
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
CIC biomaGUNE, Basque Research and Technology Alliance (BRTA), Paseo Miramon, 20014 San Sebastian, Guipuzcoa, Spain
CIBER de Enfermedades Respiratorias (CIBERES), 28029 Madrid, Spain
Search for more papers by this authorCorresponding Author
Wim M. De Borggraeve
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Joachim Demaerel
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorArmir Zogu
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorKarim Ullah
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Department of Chemistry and Technologies of Drug, Sapienza, University of Rome, P.le A. Moro 5, 00185 Rome, Italy
Search for more papers by this authorStefanos Spanopoulos
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Ermal Ismalaj
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
CIC biomaGUNE, Basque Research and Technology Alliance (BRTA), Paseo Miramon, 20014 San Sebastian, Guipuzcoa, Spain
CIBER de Enfermedades Respiratorias (CIBERES), 28029 Madrid, Spain
Search for more papers by this authorCorresponding Author
Wim M. De Borggraeve
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Joachim Demaerel
Department of Chemistry, Sustainable Chemistry for Metals and Molecules (SCM2), KU Leuven Department of Chemistry, Celestijnenlaan 200F—box 2404, B-3001 Leuven, Belgium
Search for more papers by this authorGraphical Abstract
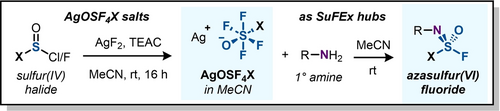
Sulfurimidoyl difluorides (RNSOF2) are excellent multidimensional SuFEx hubs, however their preparation from the scarcely available thionyl tetrafluoride (SOF4) gas limits this chemistry. Here, we propose silver pentafluorooxosulfate (AgOSF5) as a viable alternative to SOF4. Prepared by the AgF2-mediated oxidation of SOCl2, we found that the adduct produces sulfurimidoyl fluorides in high yields from primary amines, also extendable to AgOSF4CF3.
Abstract
Sulfur(VI) Fluoride Exchange (SuFEx) chemistry stands as a well-established method for swiftly constructing complex molecules in a modular fashion. An especially promising segment of this toolbox is reserved for multidimensional SuFEx hubs: three or more substituents pluggable into a singular SVI centre to make ‘beyond-linear’ clicked constructions. Sulfurimidoyl difluorides (RNSOF2) stand out as the prime example of this, however their preparation from the scarcely available thionyl tetrafluoride (SOF4) limits this chemistry to only a few laboratories with access to this gas. In this work, we identify silver pentafluorooxosulfate (AgOSF5) as a viable SuFEx hub with reactivity equal to SOF4. The AgF2-mediated oxidation of SOCl2 gives rise to the hexacoordinate AgOSF5 adduct, which in contact with primary amines produces the sulfurimidoyl fluorides in high yields. In addition, we have found this workflow to be fully extendable to the trifluoromethyl homologue, AgOSF4CF3, and we propose the use of AgOSF4X salts as a general route to azasulfur SuFEx electrophiles from commercial starting materials.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202403797-sup-0001-misc_information.pdf6.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Magre, S. Ni, J. Cornella, Angew. Chem. Int. Ed. 2022;
- 1bN. D. Ball, in Emerging Fluorinated Motifs 2020, pp. 621–674.
10.1002/9783527824342.ch21 Google Scholar
- 2
- 2aA. Barrow, C. Smedley, Q. Zheng, S. Li, J. Dong, J. Moses, Chem. Soc. Rev. 2019, 48, 4731–4758;
- 2bM. C. Giel, C. J. Smedley, J. E. Moses, in Click Chemistry, Vol. 2021/4 (Ed.: F. P. J. T. Rutjes), Thieme Verlag, Stuttgart 2022;
- 2cC. Lee, A. J. Cook, J. E. Elisabeth, N. C. Friede, G. M. Sammis, N. D. Ball, ACS Catal. 2021, 6578–6589.
- 3J. Dong, L. Krasnova, M. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2014, 53, 9430–9448.
- 4
- 4aF. Liu, C. Wang, S. Li, G. A. L. Bare, X. Chen, C. Wang, J. E. Moses, P. Wu, K. B. Sharpless, Angew. Chem. Int. Ed. 2019, 58, 8029–8033;
- 4bB. Gao, S. Li, P. Wu, J. E. Moses, K. B. Sharpless, Angew. Chem. Int. Ed. 2018, 57, 1939–1943;
- 4cS. Li, P. Wu, J. E. Moses, K. B. Sharpless, Angew. Chem. Int. Ed. 2017, 56, 2903–2908;
- 4dZ. Peng, S. Sun, M.-M. Zheng, Y. Li, X. Li, S. Li, X.-S. Xue, J. Dong, B. Gao, Nat. Chem. 2024, 16, 353–362.
- 5B.-Y. Li, K. Su, L. Van Meervelt, S. H. L. Verhelst, E. Ismalaj, W. M. De Borggraeve, J. Demaerel, Angew. Chem. Int. Ed. 2023, 62, e202305093.
- 6
- 6aS. Greed, E. L. Briggs, F. I. M. Idiris, A. J. P. White, U. Lücking, J. A. Bull, Chem. Eur. J. 2020, 26, 12533–12538;
- 6bD. D. Liang, D. E. Streefkerk, D. Jordaan, J. Wagemakers, J. Baggerman, H. Zuilhof, Angew. Chem. 2020, 132, 7564–7570; Angew. Chem. Int. Ed. 2020, 59, 7494–7500;
- 6cZ.-X. Zhang, M. C. Willis, Chem 2022, 8, 1137–1146;
- 6dS. Greed, O. Symes, J. A. Bull, Chem. Commun. 2022, 58, 5387–5390.
- 7
- 7aS. Kitamura, Q. Zheng, J. L. Woehl, A. Solania, E. Chen, N. Dillon, M. V. Hull, M. Kotaniguchi, J. R. Cappiello, S. Kitamura, V. Nizet, K. B. Sharpless, D. W. Wolan, J. Am. Chem. Soc. 2020, 142, 10899–10904;
- 7bG. J. Brighty, R. C. Botham, S. Li, L. Nelson, D. E. Mortenson, G. Li, C. Morisseau, H. Wang, B. D. Hammock, K. B. Sharpless, J. W. Kelly, Nat. Chem. 2020, 12, 906–913;
- 7cH. Mukherjee, J. Debreczeni, J. Breed, S. Tentarelli, B. Aquila, J. E. Dowling, A. Whitty, N. P. Grimster, Org. Biomol. Chem. 2017, 15, 9685–9695.
- 8
- 8aY. Chao, M. Subramaniam, K. Namitharan, Y. Zhu, V. Koolma, Z. Hao, S. Li, Y. Wang, I. Hudoynazarov, F. M. Miloserdov, H. Zuilhof, J. Org. Chem. 2023, 88, 15658–15665;
- 8bD.-D. Liang, S. P. Pujari, M. Subramaniam, M. Besten, H. Zuilhof, Angew. Chem. Int. Ed. 2022, 61, e202116158;
- 8cS. Li, G. Li, B. Gao, S. P. Pujari, X. Chen, H. Kim, F. Zhou, L. M. Klivansky, Y. Liu, H. Driss, D.-D. Liang, J. Lu, P. Wu, H. Zuilhof, J. Moses, K. B. Sharpless, Nat. Chem. 2021, 13, 858–867.
- 9A note on nomenclature: The RNS(O)F2 functional group has elsewhere been named iminosulfur oxydifluoride, which is entirely correct as well. Here, we opt to follow a common logic for azasulfur nomenclature, which is to start from the sulfonyl-containing counterpart, and using basename+[imid]+suffix for the nitrogen analogue. In this case, the parent compound is sulfuryl fluoride (SO2F2) and thus becomes sulfurimidoyl fluoride for the nitrogenated species.
- 10R. Cramer, D. D. Coffman, J. Org. Chem. 1961, 26, 4010–4014.
- 11K. Seppelt, W. Sundermeyer, Angew. Chem. Int. Ed. 1970, 9, 905.
- 12C. J. Smedley, Q. Zheng, B. Gao, S. Li, A. Molino, H. M. Duivenvoorden, B. S. Parker, D. J. D. Wilson, K. B. Sharpless, J. E. Moses, Angew. Chem. Int. Ed. 2019, 58, 4552–4556.
- 13A gas bottle can be purchased from SynQuest Labs at 795 USD/10 g (checked 16/01/2024), being the only provider globally.
- 14W. C. Smith, V. A. Engelhardt, J. Am. Chem. Soc. 1960, 82, 3838–3840.
- 15More recently, the direct fluorination of SOF2 was proposed as an alternative synthesis for SOF4: J. Dong, S. Ke, S. Li, Methods and systems for preparing SOF2 gas and SOF4 gas 2018, CN108128758A.
- 16K. Seppelt, Angew. Chem. Int. Ed. 1982, 21, 877–888.
- 17
- 17aF. Sladky, Angew. Chem. Int. Ed. 1969, 8, 523–523;
- 17bK. Seppelt, Chem. Ber. 1972, 105, 2431–2436;
- 17cF. Sladky, H. Kropshofer, O. Leitzke, J. Chem. Soc. Chem. Commun. 1973, 134–135;
- 17dH. Kropshofer, O. Leitzke, P. Peringer, F. Sladky, Chem. Ber. 1981, 114, 2644–2648;
- 17eK. Seppelt, Phosphorus Sulfur Silicon Relat. Elem. 1998, 136, 107–122;
- 17fA. Wiesner, T. W. Gries, S. Steinhauer, H. Beckers, S. Riedel, Angew. Chem. Int. Ed. 2017, 56, 8263–8266.
- 18K. Seppelt, Z. Anorg. Allg. Chem. 1977, 428, 35–42.
- 19
- 19aJ. K. Ruff, M. Lustig, Inorg. Chem. 1964, 3, 1422–1425;
- 19bM. Lustig, J. K. Ruff, Inorg. Chem. 1967, 6, 2115–2117.
- 20
- 20aW. Heilemann, R. Mews, S. Pohl, W. Saak, Chem. Ber. 1989, 122, 427–432;
- 20bP. Kirsch, W. Hierse, E. Claus, M. Kleineidam, G.-V. Röschenthaler, N. Kalinovich, New pentafluorosulfoxy derivative useful e.g. as a surfactant, a water repellent, an oil repellent, preferably in the surface modification of textiles, paper, glass, porous building materials or adsorbents, and as an antistatic agent 2012, DE102011114650A1;
- 20cR. G. Syvret, G. S. Lal, K. E. Minnich, Oxypentafluorosulfate compositions and processes for making them 2010, US7771611B2.
- 21A. Haupt, D. Duvinage, E. Lork, M. Ponomarenko, G. V. Röschenthaler, Angew. Chem. Int. Ed. 2021, 60, 17866–17870.
- 22T. Gatzenmeier, Y. Liu, M. Akamatsu, T. Okazoe, K. Nozaki, 2023, ChemRxiv preprint, DOI: 10.26434/chemrxiv-2023-jzn11.
- 23J. Demaerel, C. Veryser, W. M. De Borggraeve, React. Chem. Eng. 2020, 5, 615–631.
- 24
- 24aR. Mews, P. Kricke, I. Stahl, Z. Naturforsch. B 1981, 36, 1093–1098;
- 24bS.-L. Yu, J. M. Shreeve, J. Fluorine Chem. 1976, 7, 85–94;
- 24cR. Y. Garlyauskayte, A. V. Bezdudny, C. Michot, M. Armand, Y. L. Yagupolskii, L. M. Yagupolskii, J. Chem. Soc. Perkin Trans. 1 2002, 1887–1889;
- 24dC. S. Richards-Taylor, C. Martínez-Lamenca, J. E. Leenaerts, A. A. Trabanco, D. Oehlrich, J. Org. Chem. 2017, 82, 9898–9904;
- 24eM. Wright, C. Martínez-Lamenca, J. E. Leenaerts, P. E. Brennan, A. A. Trabanco, D. Oehlrich, J. Org. Chem. 2018, 83, 9510–9516;
- 24fB.-Y. Li, L. Voets, R. Van Lommel, F. Hoppenbrouwers, M. Alonso, S. H. L. Verhelst, W. M. De Borggraeve, J. Demaerel, Chem. Sci. 2022, 13, 2270–2279.
- 25
- 25aA.-L. Barthelemy, E. Magnier, C. R. Chim. 2018, 21, 711–722;
- 25bX. Shen, J. Hu, Eur. J. Org. Chem. 2014, 2014, 4437–4451.
- 26C. R. Pitts, N. Santschi, A. Togni, Method for preparing a polyfluorinated compound 2019, WO2019229103 A1.
- 27
- 27aF. Neese, F. Wennmohs, U. Becker, C. Riplinger, J. Chem. Phys. 2020, 152;
- 27bF. Neese, WIREs Comput. Mol. Sci. 2012, 2, 73–78.
- 28
- 28aE. Caldeweyher, S. Ehlert, A. Hansen, H. Neugebauer, S. Spicher, C. Bannwarth, S. Grimme, J. Chem. Phys. 2019, 150;
- 28bE. Caldeweyher, C. Bannwarth, S. Grimme, J. Chem. Phys. 2017, 147.
- 29F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 30F. Weigend, Phys. Chem. Chem. Phys. 2006, 8, 1057–1065.
- 31
- 31aS. Kozuch, J. M. L. Martin, Phys. Chem. Chem. Phys. 2011, 13, 20104–20107;
- 31bF. Jensen, J. Chem. Theory Comput. 2015, 11, 132–138.
- 32
- 32aF. Weigend, A. Köhn, C. Hättig, J. Chem. Phys. 2002, 116, 3175–3183;
- 32bC. Hättig, Phys. Chem. Chem. Phys. 2005, 7, 59–66.
- 33T. R. Lex, M. I. Swasy, S. Panda, B. R. Brummel, L. N. Giambalvo, K. G. Gross, C. D. McMillen, K. Kobra, W. T. Pennington, D. C. Whitehead, Tetrahedron Lett. 2020, 61, 151723.