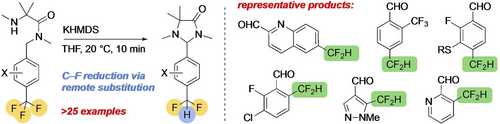
Selective Defluorination of Trifluoromethyl Substituents by Conformationally Induced Remote Substitution
Graphical Abstract
Amide derivatives of trifluoromethyl-substituted benzaldehydes, and their heterocyclic congeners, may be selectively cyclodefluorinated by a remote substitution reaction under mild conditions, giving difluoromethyl-substituted aldehydes after hydrolysis. Selective single and double defluorinations are possible using this method, which provides a versatile route to difluoromethyl arenes.
Abstract
The selective reduction of an aromatic trifluoromethyl substituent to a difluoromethyl substituent may be achieved by base-promoted elimination to form a difluoro-p-quinomethide which is trapped by an intramolecular nucleophile. High yields are obtained when the nucleophilic trap entails the conformationally favoured cyclisation of an aminoisobutyric acid (Aib) derivative. The resulting cyclised difluoromethyl-substituted arylimidazolidinone products are readily converted to versatile difluoromethyl-substituted aldehydes by reduction and hydrolysis. Defluorination is successful on a range of benzenoid (both para and ortho CF3-substituted) and heterocyclic substrates. Double defluorination may likewise be achieved sequentially, or in a single step, from an Aib dipeptide derivative.
Around 20 % of marketed drugs and 50 % of modern crop protection products contain at least one fluorine atom.1 Fluorine's unique properties can lead to enhanced metabolic stability, bioavailability, lipophilicity, membrane permeability and ultimately potency of pharmaceuticals and agrochemicals.2 Nonetheless, there is current concern about the environmental persistence of fluorinated metabolites arising from trifluoromethyl groups.3 Difluoromethyl or fluoromethyl substituents are more metabolically and environmentally degradable,3c and the unusually acidic yet hydrophobic C−H bond of the difluoromethyl group provides opportunities for unusual C−H hydrogen-bonding interactions, making it a versatile bioisostere for other functionality.4
The growing importance of the difluoromethyl group4d has led to a variety of synthetic approaches to difluoromethylated arenes,5 one of which entails selective removal of a fluorine atom from a trifluoromethyl group (Figure 1a). This approach is particularly appealing because it may allow direct replacement of trifluoromethylated synthetic intermediates with partially defluorinated analogues without significant modification of synthetic routes. However, each defluorination weakens the remaining C−F bonds,6 so selective partial defluorination is challenging to control. A limited range of trifluoromethyl arenes may be reduced using palladium and copper catalysts in the presence of Ph3SiH as a reductant,7 and selective monodefluorination of more reducible bis(trifluoromethyl)arenes can be accomplished using Mg under acidic conditions.8 Newer methods9 use photocatalytic C−F activation to broaden functional group tolerance, although each protocol is only applicable to specific arene electronics. Lennox and co-workers have developed an electrochemical procedure for the direct hydrodefluorination of electron-rich, electron-neutral and electron-deficient trifluoromethylarenes,10 a method that also provides fluoromethylarenes by double defluorination, albeit in moderate yields and selectivities.
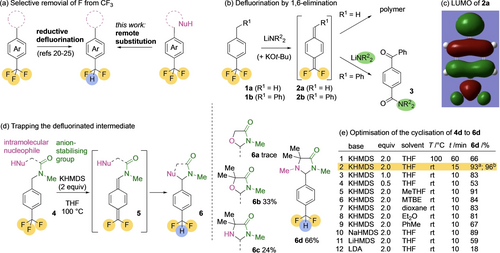
Difluoromethylarenes from trifluoromethylarenes. (a) Selective defluorination; (b) Defluorination by 1,6-elimination; (c) Calculated LUMO of proposed defluorinated intermediate 2 a; (d) Trapping the defluorinated intermediate 5. a0.2 mmol scale; b1.0 mmol scale.
In this paper we present a simple and practical method for selective removal of fluorine atoms from aromatic CF3 groups (Figure 1a). A trifluoromethylated precursor is ligated to an ‘auxiliary’ containing a nucleophilic atom (Nu). Treatment with base induces remote substitution of a fluoride leaving group, leading to a cyclised difluoromethylated product. The intramolecular substitution reaction transfers the oxidation state to another, more readily manipulated aminal function, whose reduction and cleavage returns selectively defluorinated products.
The starting point for development of the method was the report11 that deprotonation of trifluoromethyltoluene 1 a leads to monodefluorination of the trifluoromethyl group by a remote, 1,6-elimination reaction, the product of which is the unstable difluoro-p-quinomethide 2 a (Figure 1b). The mono defluorinated 2 a is reported to polymerise rapidly, but could conceivably be trapped with a nucleophile. We treated 1 b with LDA or LiNEt2, and the result suggested that nucleophilic capture of the mono-defluorinated intermediate 2 a was possible: isolated products included 3 which may arise from oxidation and hydrolysis of a 1,1-difluoroamine intermediate.
To form a stable difluoromethyl-substituted product, this nucleophilic attack needs to take place at the methylene terminus of the quinomethide intermediate 2, where DFT calculations on 2 a (Figure 1c) suggested a large LUMO coefficient. Attempts to induce regioselective attack on this potent electrophile by further modifying the substituent R1 were unfruitful, but success came with a nucleophile tethered to the trifluoromethyltoluene ring through an anion-stabilising12 amide linkage (Figure 1d). Treatment of an alcohol 4 a with KHMDS at 100 °C in THF gave traces of a defluorinated product 6 a, but using a gem-dimethyl substituent to favour cyclisation, making use of the Thorpe–Ingold effect,13 gave significantly greater success, and returned the oxazolidinone 6 b in 33 % yield under these conditions. Switching to more nucleophilic primary and secondary amines returned 6 c and 6 d in moderate to good yield. Importantly, unlike the cyclisations of 4 a–4 c, defluorinative cyclisation of 4 d to 6 d showed no evidence of polymerisation.
Optimisation of the cyclisation of 4 d with KHMDS in THF (Figure 1e, entry 1) revealed that 6 d was formed in essentially quantitative yield after only 15 minutes at room temperature, and the product could be obtained in 96 % yield on a 1.0 mmol scale (entry 2). Two equivalents of base maximised yields (entries 2–4), with alternative ether solvents or toluene also being acceptable (entries 5–9 and SI). NaHMDS was almost as effective as KHMDS; LiHMDS and LDA less so (entries 10–12).
A plausible mechanism for the cyclodefluorination of 4 d follows the pathway shown in Figure 2a. Amide 4 d can be viewed a peptide derivative of the quaternary amino acid Aib (α-aminoisobutyric acid), which induces helical domains in natural and synthetic peptide structures through a ‘conformational Thorpe–Ingold effect’.14 A similar conformational preference presumably underpins the powerful tendency of 4 d to cyclise. Amide-promoted deprotonation of the benzylic methylene group by hexamethyldisilazide and loss of fluoride generates a difluoroquinomethide intermediate 5 d. This reactive species captures the pendent amine [presumably in neutral form, given the relative pKa of secondary amines (~36 in THF) and hexamethyldisilazane (26 in THF)15] and undergoes rapid cyclisation to the α, α-difluorobenzyl anion 6 d−. This anion is protonated by the hexamethyldisilazane formed in situ to give the product 6 d.
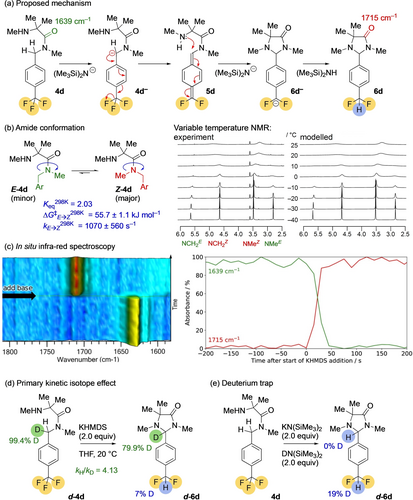
The defluorination mechanism. (a) Proposed mechanism; (b) Conformational studies of the amide function by variable temperatue NMR; (c) In situ infra-red studies; (d) The primary kinetic isotope effect; (e) Trapping the product anion with deuterium.
Additional experiments elucidated further mechanistic details. The starting material 4 d is a 2.03 : 1 mixture of Z and E rotamers about the amide C−N bond (Figure 2b), but VT NMR (Figure 2c) reveals that these interconvert on the millisecond timescale at room temperature. Deprotonation of amides with non-coordinating bases such as KHMDS is expected16 to favour removal of a proton trans to a carbonyl group, and an in situ IR study (Figure 2d) suggested that this deprotonation is rate-determining, and complete within seconds. Direct and essentially instantaneous transformation of the starting material 4 d (C=O stretch 1639 cm−1) to the product 6 d (C=O stretch 1715 cm−1) proceeds through no detectable intermediates.
The reaction exhibits a significant primary kinetic isotope effect (Figure 2d). Amide d-4 d, mono-deuterated at its benzylic position (99.4 % d1) was subjected to the standard reaction conditions. The product retains 79.9 % deuterium at this position, a value indicating a primary kinetic isotope effect of kH/kD=4.13 at 20 °C. This is consistent with a rate-determining C−H deprotonation step leading towards 4 d− which may or may not be concerted with rapid loss of fluoride to give 5 d: in other words either an E1cbirr or an E2 elimination. An irreversible deprotonation is consistent with an observation (Figure 2e) that carrying out the reaction in the presence of N-deuterohexamethyldisilazane returned no product deuterated α to nitrogen. Some deuterium incorporated in the difluoromethyl group suggests that the final protonation step is performed by hexamethyldisilazane (which is generated during the reaction, partially deuterated from a deuterated starting material). 19F NMR of the crude product mixture identified Me3SiF as a second fluorine-containing product (see SI), suggesting a further role for hexamethyldisilazane in trapping the departing fluoride ion.
The overall consequence of the transformation of 4 d to 6 d is the 1,6-telesubstitution of fluoride by an amino group, with transfer of oxidation state from the CF3 group to the carbon atom α to the amide nitrogen. The reaction has the potential for application as a method for the otherwise challenging selective monodefluorination of aromatic trifluoromethyl groups, provided that the products can be readily transformed into practical synthetic intermediates. Removal of the Aib defluorination ‘auxiliary’ was achieved by reduction of the amide group of 6 d (=10 a, Figure 3a) with DIBAL, followed by hydrolysis with aqueous HCl, which gave the product aldehyde 11 a in 87 % yield.
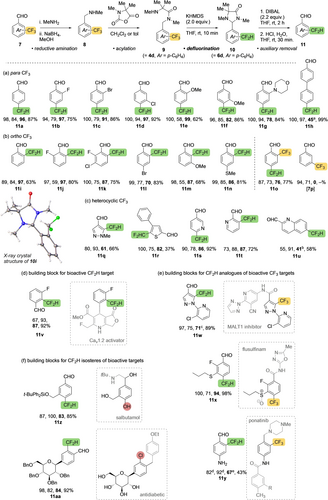
Scope and applications of the reaction. Isolated yields are given for each transformation in the four-step sequence. a100 °C, μw, 1 h; b100 °C, μw, 30 min; c−10 °C, 1 h; damine protected as the N,N-diallyl amine; edeprotected using Pd(PPh3)4, N,N-dimethylbarbituric acid, CH2Cl2.
Given that the starting material 4 d (=9 a, Figure 3a) for the cyclisation may be formed from trifluoromethylbenzaldehyde 7 a by reductive amination to 8 a and acylation with an Aib-derived N-carboxyanhydride (Figure 3a), the overall transformation of 7 to 11 constitutes a vicarious reduction of the CF3 group, transferring oxidation from one C−F bond to the aldehyde carbon during the cyclodefluorination. This operationally simple four-step method—reductive amination, acylation, cyclisation and reductive hydrolysis—was successfully extended to other trifluoromethylated aromatic aldehydes as illustrated in Figure 3, in which the yields of each of the four steps are given for every entry, with the defluorination yield (9→10) in bold.
Defluorination was generally high yielding with diverse 4-trifluoromethyl substituted rings, giving 4-difluoromethylbenzaldehydes 11 bearing additional substituents including halogens (11 b,c,d) and other heteroatoms (11 e,f,g). Although slower and lower-yielding, defluorination was also successful through a more remote 1,10-elimination, giving difluoromethylbiphenyl 11 h. The proposed mechanism (Figure 2a, via 4 d−) for defluorination requires a 1,n relationship between the aldehyde and the CF3 group where n is even. Thus, while meta-substituted trifluoromethylbenzaldehyde derivative 7 p (Figure 4b) failed to defluorinate, variously substituted ortho-difluoromethylbenzaldehydes 11 i,j,k,l,m and n were formed through a 1,4-telesubstitution under similar conditions to their para congeners (Figure 3b). Starting from a 2,4-bis(trifluoromethyl)benzaldehyde, the 4-trifluoromethyl group was defluorinated preferentially (11 o), perhaps because an alternative 1,4-elimination entails a greater increase in A1,3 strain. The X-ray crystal structure of 10 i17 reveals an intramolecular N⋅⋅⋅HCF2 hydrogen bond (2.4 Å) between the amine and the newly formed ortho CHF2 group.
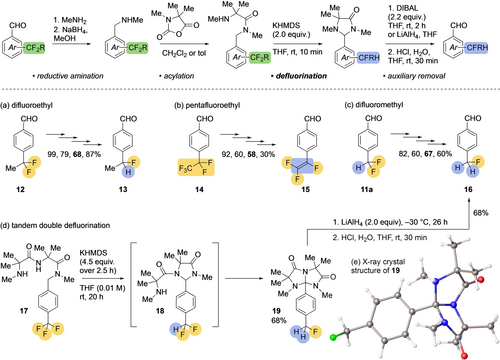
Defluorination of difluoroalkyl groups. Isolated yields are given for each transformation in the four-step sequence.
Defluorination was also successful with heterocyclic trifluoromethyl substituents (Figure 3c). Thus, 1,4-telesubstitution gave difluoromethylpyrazole 11 q, and 1,6-telesubstitution gave difluoromethylthiophene 11 r. 1,4- and 1,6-telesubstitutions gave 2- and 3-difluoromethylpyridine derivatives 11 s and 11 t and 1,8-telesubstitution gave 6-difluoromethylquinoline 11 u.
The practicality and broader utility of the method was demonstrated in the preparation of a known precursor to an active difluoromethyl-containing target (Figure 3d), and of difluoromethyl analogues of active pharmaceutical or agrochemical agents (Figure 3e,f). Thus 11 v is a precursor to a known calcium channel activator,18 and 11 w, 11 x and 11 y constitute difluoromethyl analogues of trifluoromethyl-containing precursors to a MALT1 inhibitor,19 the herbicide flusulfinam,20 and the drug ponatinib21 (Figure 3e). Difluoromethyl groups provide unusual isosteres for halogens or phenols,4d, 22 offering localised electron density and a moderately acidic C−H atom (Figure 3f). 11 z is a difluoromethyl isosteric replacement of the phenolic hydroxyl group in an aldehyde precursor to salbutamol, and 11 aa provides a difluoromethyl isosteric replacement of the chloro substituent of an antidiabetic agent.23
The method was extended more broadly to the defluorination of difluoroalkyl substituents to form fluoroalkyl products (Figure 4). For example, using the same strategy with 4-(1,1-difluoroethyl)benzaldehyde 12 (Figure 4a) gave the 4-(1-fluoroethyl)benzaldehyde 13 in good yield. With the pentafluoroethyl group of 14 (Figure 4b) an additional 1,2-elimination of HF occurs, forming trifluorostyrene 15 by formal reductive extrusion of F2 from 14.
It is also possible to defluorinate the difluoromethylbenzaldeyde product 11 a again: fluoromethylbenzaldehyde 16 was formed after the same four steps (Figure 4c). However, double defluorination of a trifluoromethyl substituent could in principle also be achieved in a single step by modification of the ‘auxiliary’ to include a second nucleophile. With this aim, trifluoromethylbenzaldehyde was ligated to two Aib residues to make the Aib dipeptide 17 (Figure 4d: see Supporting Information for details). Slow addition of KHMDS at 50 °C to a solution of 17 in THF led to the triazabicyclic 19 in 68 % yield. The remarkable structure of 19 was confirmed by X-ray crystallography,17 and is presumably formed by two successive remote substitutions that allow the conformationally preorganised dipeptide to wrap itself around the benzylic carbon. Removal of the auxiliary was achieved by reduction of 19 with LiAlH4, followed by hydrolysis with aqueous HCl, yielding aldehyde 16 in 68 % yield.
In summary, the remote substitution of a fluoride group by an intramolecular nitrogen nucleophile allows the selective removal of a single fluorine atom from aromatic trifluoromethyl and difluoroalkyl substituents. A standard four-step procedure (reductive amination, acylation, cyclisation and hydrolysis) provides a protocol for the selective reduction of trifluoromethyl-substituted aromatic aldehydes to their difluoromethyl-substituted equivalents, provided the trifluoromethyl group and aldehyde carbonyl have a 1,n relationship where n is even.
Supporting Information
Full experimental details and characterisation data. Additional references are cited within the Supporting Information.24-75
Acknowledgments
This work was supported by the EPSRC through the Bristol EPSRC Centre for Doctoral Training in Technology-Enhanced Chemical Synthesis (EP/S024107/1) and the EPSRC Centre for Doctoral Training in Catalysis (EP/L016443/1), the ERC (AdG DOGMATRON 883786), and an RSC Undergraduate Bursary (U23-2866881622). We thank Dr Hazel Sparkes for determining the X-ray crystal structures of 10 i and 19.
Conflict of interests
The authors declare no conflict of interest.