Enzymatic Synthesis of Noncanonical α-Amino Acids Containing γ-Tertiary Alcohols
Corresponding Author
Rui Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorChenghua Zhang
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308 China
School of Pharmacy, North Sichuan Medical College, Nanchong, 637100 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJiamu Tan
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorYifan He
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorDan Zhuo
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorJingxuan Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorZhenzhen Luo
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorQiaoqiao Li
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorJiaying Yao
Graduate School, Jiangxi University of Chinese Medicine, Nanchang, 330004 China
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorChangqiang Ke
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorChunping Tang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorYang Ye
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Shijun He
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Xiang Sheng
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308 China
National Center of Technology Innovation for Synthetic Biology, National Engineering Research Center of Industrial Enzymes and Key Laboratory of Engineering Biology for Low-Carbon Manufacturing, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Cangsong Liao
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Rui Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorChenghua Zhang
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308 China
School of Pharmacy, North Sichuan Medical College, Nanchong, 637100 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJiamu Tan
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorYifan He
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
Search for more papers by this authorDan Zhuo
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorJingxuan Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorZhenzhen Luo
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorQiaoqiao Li
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
Search for more papers by this authorJiaying Yao
Graduate School, Jiangxi University of Chinese Medicine, Nanchang, 330004 China
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
Search for more papers by this authorChangqiang Ke
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorChunping Tang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorYang Ye
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Shijun He
Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Xiang Sheng
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, 300308 China
National Center of Technology Innovation for Synthetic Biology, National Engineering Research Center of Industrial Enzymes and Key Laboratory of Engineering Biology for Low-Carbon Manufacturing, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Cangsong Liao
State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, 210023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorGraphical Abstract
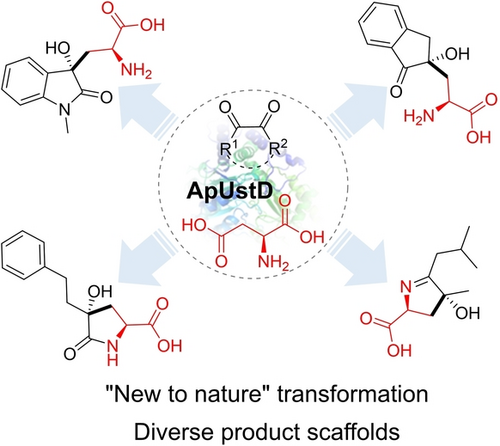
An enzymatic decarboxylative aldol reaction was developed for the synthesis of noncanonical α-amino acids containing γ-tertiary alcohol groups. The modularity of the electrophile enabled access to four classes of ncAAs with high efficiency as well as excellent regioselectivity and stereoselectivity.
Abstract
Noncanonical amino acids (ncAAs) containing tertiary alcohols are valuable as precursors of natural products and active pharmaceutical ingredients. However, the assembly of such ncAA scaffolds from simple material by C−C bond formation remains a challenging task due to the presence of multiple stereocenters and large steric hindrance. In this study, we present a novel solution to this problem through highly selective enzymatic decarboxylative aldol addition. This method allows for the streamlined assembly of multifunctionalized ncAAs with γ-tertiary alcohols from readily available materials, such as L-aspartatic acid and isatins, vicinal diones and keto esters. The modularity of electrophiles furnished four classes of ncAAs with decent efficiency as well as excellent site and stereocontrol. Computational modeling was employed to gain detailed insight into the catalytic mechanism and to provide a rationale for the observed selectivities. The method offers a single-step approach to producing multifunctionalized ncAAs, which can be directly utilized in peptide synthesis and bioactivity assessment.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318550-sup-0001-3ac.cif156.8 KB | Supporting Information |
| anie202318550-sup-0001-3b.cif380.8 KB | Supporting Information |
| anie202318550-sup-0001-3k.cif429.2 KB | Supporting Information |
| anie202318550-sup-0001-3p.cif360.2 KB | Supporting Information |
| anie202318550-sup-0001-9.cif191.8 KB | Supporting Information |
| anie202318550-sup-0001-misc_information.pdf11.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. A. T. Blaskovich, J. Med. Chem. 2016, 59, 10807–10836;
- 1bM. Muttenthaler, G. F. King, D. J. Adams, P. F. Alewood, Nat. Rev. Drug Discovery 2021, 20, 309–325.
- 2Y. Ding, J. P. Ting, J. Liu, S. Al-Azzam, P. Pandya, S. Afshar, Amino Acids 2020, 52, 1207–1226.
- 3J. L. Hickey, D. Sindhikara, S. L. Zultanski, D. M. Schultz, ACS Med. Chem. Lett. 2023, 14, 557–565.
- 4
- 4aC. T. Walsh, R. V. O′Brien, C. Khosla, Angew. Chem. Int. Ed. 2013, 52, 7098–7124;
- 4bR. D. Sussmuth, A. Mainz, Angew. Chem. Int. Ed. 2017, 56, 3770–3821.
- 5Amino Acid Market Research Report by Product Type (Creatine, Glutamine, L-glutamate), Source (Animal-Based, Plant-Based, Synthetic), Application - Global Forecast 2023–2030.
- 6M. W. Pennington, B. Zell, C. J. Bai, Med. Drug Discovery 2021, 9, 100071.
- 7
- 7aF. J. Aguilar Troyano, K. Merkens, K. Anwar, A. Gomez-Suarez, Angew. Chem. Int. Ed. 2021, 60, 1098–1115;
- 7bV. A. Larionov, N. V. Stoletova, V. I. Maleev, Adv. Synth. Catal. 2020, 362, 4325–4367.
- 8N. Richy, D. Sarraf, X. Marechal, N. Janmamode, R. Le Guevel, E. Genin, M. Reboud-Ravaux, J. Vidal, Eur. J. Med. Chem. 2018, 145, 570–587.
- 9R. Sakai, H. Kamiya, M. Murata, K. Shimamoto, J. Am. Chem. Soc. 1997, 119, 4112–4116.
- 10R. Vleggaar, L. G. J. Ackerman, P. S. Steyn, J. Chem. Soc. Perkin Trans. 1 1992, 3095–3098.
- 11
- 11aS. Kortet, A. Claraz, P. M. Pihko, Org. Lett. 2020, 22, 3010–3013;
- 11bX.-S. Yin, W.-Y. Qi, B.-F. Shi, Chem. Sci. 2021, 12, 13137–13143.
- 12
- 12aY. P. Xue, C. H. Cao, Y. G. Zheng, Chem. Soc. Rev. 2018, 47, 1516–1561;
- 12bP. J. Almhjell, C. E. Boville, F. H. Arnold, Chem. Soc. Rev. 2018, 47, 8980–8997;
- 12cE. Alfonzo, A. Das, F. H. Arnold, Curr. Opin. Green Sustain. Chem. 2022, 38, 100701;
- 12dC. Liao, F. P. Seebeck, Angew. Chem. Int. Ed. 2020, 59, 7184–7187.
- 13
- 13aM. Dick, N. S. Sarai, M. W. Martynowycz, T. Gonen, F. H. Arnold, J. Am. Chem. Soc. 2019, 141, 19817–19822;
- 13bV. Laurent, L. Gourbeyre, A. Uzel, V. Hélaine, L. Nauton, M. Traïkia, V. de Berardinis, M. Salanoubat, T. Gefflaut, M. Lemaire, C. Guérard-Hélaine, ACS Catal. 2020, 10, 2538–2543.
- 14Y. Ye, A. Minami, Y. Igarashi, M. Izumikawa, M. Umemura, N. Nagano, M. Machida, T. Kawahara, K. Shin-Ya, K. Gomi, H. Oikawa, Angew. Chem. Int. Ed. 2016, 55, 8072–8075.
- 15R. Zhang, J. Tan, Z. Luo, H. Dong, N. Ma, C. Liao, Catal. Sci. Technol. 2021, 11, 7380–7385.
- 16
- 16aJ. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki, S. Komatsubara, J. Org. Chem. 2000, 65, 990–995;
- 16bY.-Q. Tang, I. Sattler, R. Thiericke, S. Grabley, X.-Z. Feng, Eur. J. Org. Chem. 2001, 261–267.
- 17J. M. Ellis, M. E. Campbell, P. Kumar, E. P. Geunes, C. A. Bingman, A. R. Buller, Nat. Catal. 2022, 5, 136–143.
- 18Y. L. Du, K. S. Ryan, Nat. Prod. Rep. 2019, 36, 430–457.
- 19W. Zheng, Z. Pu, L. Xiao, G. Xu, L. Yang, H. Yu, J. Wu, Angew. Chem. Int. Ed. 2023, 62, e202213855.
- 20M. H. M. Olsson, C. R. Søndergard, M. Rostkowski, J. H. Jensen, J. Chem. Theory Comput. 2011, 7, 525–537.
- 21
- 21aJ. Mongan, D. A. Case, J. A. McCammon, J. Comput. Chem. 2004, 25, 2038–2048;
- 21bJ. M. Swails, D. M. York, A. E. Roitberg, J. Chem. Theory Comput. 2014, 10, 1341–1352.
- 22
- 22aX. Sheng, F. Himo, Acc. Chem. Res. 2023, 56, 938–947;
- 22bF. Himo, S. P. de Visser, Commun. Chem. 2022, 5, 29;
- 22cP. E. M. Siegbahn, RSC Adv. 2021, 11, 3495–3508;
- 22dX. Sheng, M. Kazemi, F. Planas, F. Himo, ACS Catal. 2020, 10, 6430–6449;
- 22eF. Himo, J. Am. Chem. Soc. 2017, 139, 6780–6786.
- 23Deposition numbers 2220473 (for 3 ac), 2220464 (for 3 b), 2220465 (for 3 k), 2220470 (for 3 p), and 2220471 (for 9) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 24A. Ciesielska, M. Matyjek, K. Kwiatkowska, Cell. Mol. Life Sci. 2021, 78, 1233–1261.
- 25D. M. Mosser, J. P. Edwards, Nat. Rev. Immunol. 2008, 8, 958–969.
- 26R. Crawshaw, A. E. Crossley, L. Johannissen, A. J. Burke, S. Hay, C. Levy, D. Baker, S. L. Lovelock, A. P. Green, Nat. Chem. 2022, 14, 313–320.
- 27
- 27aV. Hélaine, C. Gastaldi, M. Lemaire, P. Clapés, C. Guérard-Hélaine, ACS Catal. 2021, 12, 733–761;
- 27bM. Liu, D. Wei, Z. Wen, J.-B. Wang, Front. Bioeng. Biotechnol. 2022, 9, 653682.