A Double-ligand Chelating Strategy to Iron Complex Anolytes with Ultrahigh Cyclability for Aqueous Iron Flow Batteries
Shaocong Wang
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorLong Ma
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorShiyang Niu
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorShibo Sun
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorYong Liu
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yuanhui Cheng
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorShaocong Wang
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorLong Ma
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorShiyang Niu
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorShibo Sun
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorYong Liu
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yuanhui Cheng
State Key Laboratory of Organic-Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing, 100029 P. R. China
Search for more papers by this authorGraphical Abstract
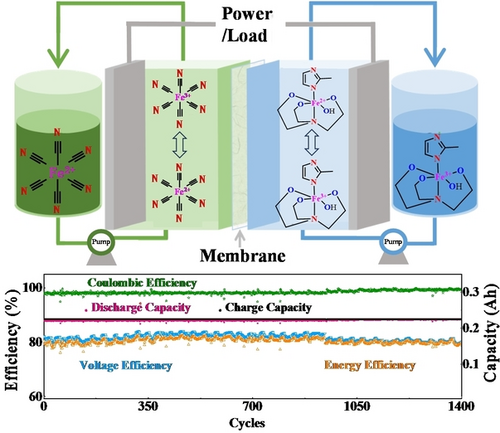
A double-ligand chelating strategy was proposed to design Fe(TEA)MM complex as anolyte materials with high binding energy, robust coordination structure, highly electrochemical activity and good reversibility. All iron flow batteries based on Fe(TEA)MM anolytes and Fe(CN)6 catholytes reach a low capital cost of $ 33.2 kWh−1 and record-breaking cycling stability without detectable energy efficiency and capacity decay during 1400 cycles.
Abstract
Aqueous all-iron flow batteries (AIFBs) are attractive for large-scale and long-term energy storage due to their extremely low cost and safety features. To accelerate commercial application, a long cyclable and reversible iron anolyte is expected to address the critical barriers, namely iron dendrite growth and hydrogen evolution reaction (HER). Herein, we report a robust iron complex with triethanolamine (TEA) and 2-methylimidazole (MM) double ligands. By introducing two ligands into one iron center, the binding energy of the complex increases, making it more stable in the charge-discharge reactions. The Fe(TEA)MM complex achieves reversible and stable redox between Fe3+ and Fe2+, without metallic iron growth and HER. AIFBs based on this anolyte perform a high energy efficiency of 80.5 % at 80 mA cm−2 and exhibit a record durability among reported AIFBs. The efficiency and capacity retain nearly 100 % after 1,400 cycles. The capital cost of this AIFB is $ 33.2 kWh−1 (e.g., 20 h duration), cheaper than Li-ion battery and vanadium flow battery. This double-ligand chelating strategy not only solves the current problems faced by AIFBs, but also provides an insight for further improving the cycling stability of other flow batteries.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202316593-sup-0001-misc_information.pdf6.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Wang, J. Song, D. Shi, Nat. Commun. 2023, 14, 6434.
- 2A. Cherp, V. Vinichenko, J. Tosun, J. A. Gordon, J. Jewell, Nat. Energy 2021, 6, 742–754.
- 3
- 3aP. Veers, K. Dykes, E. Lantz, S. Barth, C. L. Bottasso, O. Carlson, A. Clifton, J. Green, P. Green, H. Holttinen, D. Laird, V. Lehtomaki, J. K. Lundquist, J. Manwell, M. Marquis, C. Meneveau, P. Moriarty, X. Munduate, M. Muskulus, J. Naughton, L. Pao, J. Paquette, J. Peinke, A. Robertson, J. Sanz Rodrigo, A. M. Sempreviva, J. C. Smith, A. Tuohy, R. Wiser, Science 2019, 366, 443;
- 3bL. Liu, G. He, M. Wu, G. Liu, H. Zhang, Y. Chen, J. Shen, S. Li, Nat. Energy 2023, 8, 870–880;
- 3cD. L. Woodard, A. Snyder, J. R. Lamontagne, C. Tebaldi, J. Morris, K. V. Calvin, M. Binsted, P. Patel, Earth's Future 2023, 11, e2022EF003442.
- 4
- 4aP. Ruan, S. Liang, B. Lu, H. J. Fan, J. Zhou, Angew. Chem. Int. Ed. 2022, 61, e202200598;
- 4bD. G. Kwabi, Y. Ji, M. J. Aziz, Chem. Rev. 2020, 120, 6467–6489.
- 5
- 5aC. Choi, S. Kim, R. Kim, Y. Choi, S. Kim, H.-y. Jung, J. H. Yang, H.-T. Kim, Renewable Sustainable Energy Rev. 2017, 69, 263–274;
- 5bC. Sun, H. Zhang, ChemSusChem 2022, 15, e202101798.
- 6
- 6aH. Chen, X. Zhang, S. Zhang, S. Wu, F. Chen, J. Xu, Chem. Eng. J. 2022, 429, 132043;
- 6bJ.-E. Jang, R.-a. Kim, S. Jayasubramaniyan, C. Lee, J. Choi, Y. Lee, S. Kang, J. Ryu, S. W. Lee, J. Cho, D. W. Lee, H.-K. Song, W. Choe, D.-H. Seo, H.-W. Lee, Adv. Energy Mater. 2023, 13, 2300707;
- 6cA. Khor, P. Leung, M. R. Mohamed, C. Flox, Q. Xu, L. An, R. G. A. Wills, J. R. Morante, A. A. Shah, Mater. Today Energy 2018, 8, 80–108.
- 7M. Skyllas-Kazacos, ECS Trans. 2019, 89, 29.
- 8
- 8aY. H. Wen, H. M. Zhang, P. Qian, H. T. Zhou, P. Zhao, B. L. Yi, Y. S. Yang, Electrochim. Acta 2006, 51, 3769–3775;
- 8bS. E. Waters, B. H. Robb, M. P. Marshak, ACS Energy Lett. 2020, 5, 1758–1762.
- 9
- 9aB. Yang, J. He, G. Zhang, Vol. 3, Metall. Min. Ind. 2021, pp. 33–58;
- 9bU. S. G. Survey, Vol. 35, USGS Publications Warehouse. 2023, pp. 98–101.
- 10J. Chullipparambil Balakrishnan, M. P. Peter, D. D. Kombarakaran, J. A. Kunjilona, J. V. Thomas, ChemistrySelect 2022, 7, e202201222.
- 11L. W. Hruska, R. F. Savinell, J. Electrochem. Soc. 1981, 128, 18.
- 12L. F. Arenas, A. Loh, D. P. Trudgeon, X. Li, C. Ponce de León, F. C. Walsh, Renewable Sustainable Energy Rev. 2018, 90, 992–1016.
- 13A. Dinesh, S. Olivera, K. Venkatesh, M. S. Santosh, M. G. Priya, Inamuddin, A. M. Asiri, H. B. Muralidhara, Environ. Chem. Lett. 2018, 16, 683–694.
- 14S. Belongia, X. Wang, X. Zhang, Adv. Funct. Mater. 2023, 2302077.
- 15
- 15aK. Gong, F. Xu, J. B. Grunewald, X. Ma, Y. Zhao, S. Gu, Y. Yan, ACS Energy Lett. 2016, 1, 89–93;
- 15bN. Arroyo-Currás, J. W. Hall, J. E. Dick, R. A. Jones, A. J. Bard, J. Electrochem. Soc. 2015, 162, A378;
- 15cA. Lê, D. Floner, T. Roisnel, O. Cador, L. Chancelier, F. Geneste, Electrochim. Acta 2019, 301, 472–477.
- 16A. S. N. Murthy, T. Srivastava, J. Power Sources 1989, 27, 119–126.
- 17Y. Zhen, C. Zhang, J. Yuan, Y. Zhao, Y. Li, J. Power Sources 2020, 445, 227331.
- 18X. Liu, T. Li, Z. Yuan, X. Li, J. Energy Chem. 2022, 73, 445–451.
- 19C. Noh, Y. Chung, Y. Kwon, Chem. Eng. J. 2021, 405, 126966.
- 20M. Shin, C. Noh, Y. Chung, Y. Kwon, Chem. Eng. J. 2020, 398,125631.
- 21M. Shin, C. Noh, Y. Kwon, Int. J. Energy Res. 2022, 46, 6866–6875.
- 22K. L. Hawthorne, J. S. Wainright, R. F. Savinell, J. Electrochem. Soc. 2014, 161, A1662–A1671.
- 23Y. T. Lin, S. N. Lai, J. M. Wu, Adv. Mater. 2020, 32, 2002875.
- 24
- 24aT. E. Westre, P. Kennepohl, J. G. DeWitt, B. Hedman, K. O. Hodgson, E. I. Solomon, J. Am. Chem. Soc. 1997, 119, 6297–6314;
- 24bA. P. Grosvenor, J. E. Greedan, J. Phys. Chem. C 2009, 113, 11366–11372.
- 25J. A. Sigrist, M. W. Gaultois, A. P. Grosvenor, J. Phys. Chem. C 2011, 115, 1908–1912.
- 26E. R. Aluri, A. P. Grosvenor, RSC Adv. 2015, 5, 80939–80949.
- 27D. Dong, T. Wang, Y. Sun, J. Fan, Y.-C. Lu, Nat. Sustainability 2023, 6, 1474–1484.
- 28D. Dickson, F. Berry, Mössbauer Spectroscopy, Vol. 4, Cambridge University Press, Cambridge, 1986, pp. 70–142.
- 29
- 29aC. Huang, Y. Zhu, X. Wang, X. Liu, J. Wang, T. Zhang, J. Catal. 2017, 347, 9–20;
- 29bU. I. Kramm, M. Lefèvre, N. Larouche, D. Schmeisser, J.-P. Dodelet, J. Am. Chem. Soc. 2014, 136, 978–985.
- 30M. Macino, A. J. Barnes, S. M. Althahban, R. Qu, E. K. Gibson, D. J. Morgan, S. J. Freakley, N. Dimitratos, C. J. Kiely, X. Gao, A. M. Beale, D. Bethell, Q. He, M. Sankar, G. J. Hutchings, Nat. Catal. 2019, 2, 873–881.
- 31
- 31aF. Jiang, X. Zhou, D. Guo, Electrochim. Acta 2023, 445, 142064;
- 31bD. Anarghya, M. S. Anantha, K. Venkatesh, M. S. Santosh, M. G. Priya, H. B. Muralidhara, J. Electroanal. Chem. 2020, 878, 114577;
- 31cM. O. Bamgbopa, Y. Shao-Horn, R. Hashaikeh, S. Almheiri, Electrochim. Acta 2018, 267, 41–50;
- 31dP. Schröder, N. Aguilo-Aguayo, D. Obendorf, T. Bechtold, Electrochim. Acta 2022, 430, 141042;
- 31eC. Noh, Y. Chung, Y. Kwon, J. Power Sources 2021, 495, 229799;
- 31fH. Lim, M. Shin, C. Noh, E. Koo, Y. Kwon, K. Y. Chung, Korean J. Chem. Eng. 2022, 39, 3146–3154;
- 31gY. Song, H. Yan, H. Hao, Z. Liu, C. Yan, A. Tang, Small 2022, 18, 2204356;
- 31hY. Song, K. Zhang, X. Li, C. Yan, Q. Liu, A. Tang, J. Mater. Chem. A 2021, 9, 26354–26361.
- 32W. Zhang, J. Lu, Z. Guo, Mater. Today 2021, 50, 400–417.
- 33R. M. Darling, Curr. Opin. Chem. Eng. 2022, 37, 100855.
- 34T. Liu, X. Wei, Z. Nie, V. Sprenkle, W. Wang, Adv. Energy Mater. 2016, 6, 1501449.
- 35Z. Li, M. S. Pan, L. Su, P.-C. Tsai, A. F. Badel, J. M. Valle, S. L. Eiler, K. Xiang, F. R. Brushett, Y.-M. Chiang, Joule 2017, 1, 306–327.
- 36I. Gyuk, M. Johnson, J. Vetrano, K. Lynn, W. Parks, R. Handa, L. Kannberg, S. Hearne, K. Waldrip, R. Braccio, “Grid Energy Storage”, can be found under https://www.energy.gov/oe/articles/grid-energy-storage-december-2013, 2013 (accessed: 2023.10.31).
- 37T. Li, F. Xing, T. Liu, J. Sun, D. Shi, H. Zhang, X. Li, Energy Environ. Sci. 2020, 13, 4353–4361.
- 38Y. K. Zeng, T. S. Zhao, L. An, X. L. Zhou, L. Wei, J. Power Sources 2015, 300, 438–443.
- 39
- 39aM. Zheng, J. Sun, C. J. Meinrenken, T. Wang, J. Electrochem. Energy Convers. Storage 2019, 16, 021001;
- 39bT. Janoschka, N. Martin, U. Martin, C. Friebe, S. Morgenstern, H. Hiller, M. D. Hager, U. S. Schubert, Nature 2015, 527, 78–81.
- 40
- 40aD. C. Koningsberger, R. Prins, Nachr. Chem. Tech. Lab. 1988, 92, 229–330;
- 40bJ. Rehr, R. Albers, Rev. Mod. Phys. 2000, 72, 621–654;
- 40cB. Ravel, M. Newville, J. Synchrotron Radiat. 2005, 12, 537–541.
- 41
- 41aA. J. Bard, L. R. Faulkner, Electrochemical Method: Fundamentals and Applications, Wiley, Hoboken, 2010);
- 41bJ. Zheng, L. A. Archer, Sci. Adv. 2021, 7, eabe0219.