Stereocontrolled Synthesis of α-3-Deoxy-d-manno-oct-2-ulosonic Acid (α-Kdo) Glycosides Using C3-p-Tolylthio-Substituted Kdo Donors: Access to Highly Branched Kdo Oligosaccharides
Ao Sun
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (lead), Methodology (lead), Writing - original draft (lead)
Search for more papers by this authorZipeng Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (supporting), Methodology (supporting), Validation (lead)
Search for more papers by this authorYuchao Wang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (supporting), Methodology (supporting), Visualization (supporting)
Search for more papers by this authorDr. Shuai Meng
Key Laboratory of Tropical Biological Resources of Ministry of Education, School of Pharmaceutical Sciences, College of Marine Science, Hainan University, Haikou, 570228 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorDr. Xiao Zhang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorProf. Dr. Xiangbao Meng
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorDr. Shuchun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorCorresponding Author
Dr. Zhongtang Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Software (lead)
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhongjun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Conceptualization (lead), Funding acquisition (lead), Project administration (lead), Resources (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorAo Sun
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (lead), Methodology (lead), Writing - original draft (lead)
Search for more papers by this authorZipeng Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (supporting), Methodology (supporting), Validation (lead)
Search for more papers by this authorYuchao Wang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Investigation (supporting), Methodology (supporting), Visualization (supporting)
Search for more papers by this authorDr. Shuai Meng
Key Laboratory of Tropical Biological Resources of Ministry of Education, School of Pharmaceutical Sciences, College of Marine Science, Hainan University, Haikou, 570228 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorDr. Xiao Zhang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorProf. Dr. Xiangbao Meng
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorDr. Shuchun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Data curation (equal), Writing - review & editing (supporting)
Search for more papers by this authorCorresponding Author
Dr. Zhongtang Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Software (lead)
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhongjun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Contribution: Conceptualization (lead), Funding acquisition (lead), Project administration (lead), Resources (lead), Supervision (lead), Writing - review & editing (lead)
Search for more papers by this authorGraphical Abstract
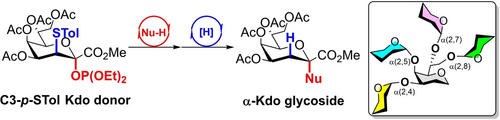
A modified donor has been developed for the glycosylation of 3-deoxy-d-manno-2-octulosonic acid (Kdo). The high reactivity and wide substrate scope of the donor enabled the synthesis of a range of Kdo-containing glycosides with complete α-stereoselectivity without the formation of 2,3-ene by-products. Several natural oligosaccharides were synthesized by a stepwise or one-pot process, including a highly branched Kdo pentasaccharide.
Abstract
3-Deoxy-d-manno-oct-2-ulosonic acid (Kdo) is an eight-carbon monosaccharide found widely in bacterial lipopolysaccharides (LPSs) and capsule polysaccharides (CPSs). We developed an indirect method for the stereoselective synthesis of α-Kdo glycosides with a C3-p-tolylthio-substituted Kdo phosphite donor. The presence of the p-tolylthio group enhanced the reactivity, suppressed the formation of elimination by-products (2,3-enes), and provided complete α-stereocontrol. A variety of Kdo α-glycosides were synthesized by our method in excellent yields (up to 98 %). After glycosylation, the p-tolylthio group can be efficiently removed by free-radical reduction. Subsequently, the orthogonality of the phosphite donor and thioglycoside donor was demonstrated by the one-pot synthesis of a trisaccharide in Helicobacter pylori and Neisseria meningitidis LPS. Moreover, an efficient total synthesis route to the challenging 4,5-branched Kdo trisaccharide in LPSs from several A. baumannii strains was highlighted. To demonstrate the high reactivity of our approach further, the highly crowded 4,5,7,8-branched Kdo pentasaccharide was synthesized as a model molecule for the first time. Additionally, the reaction mechanism was investigated by DFT calculations.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202313985-sup-0001-misc_information.pdf37.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. M. Unger, Adv. Carbohydr. Chem. Biochem. 1981, 38, 323–388;
- 1bL. Cipolla, L. Gabrielli, D. Bini, L. Russo, N. Shaikh, Nat. Prod. Rep. 2010, 27, 1618–1629.
- 2
- 2aM. A. Schmidt, K. Jann, Eur. J. Biochem. 1983, 131, 509–517;
- 2bO. Holst, FEMS Microbiol. Lett. 2007, 271, 3–11;
- 2cC. R. H. Raetz, C. Whitfield, Annu. Rev. Biochem. 2002, 71, 635–700.
- 3
- 3aB. S. Park, D. H. Song, H. M. Kim, B.-S. Choi, H. Lee, J.-O. Lee, Nature 2009, 458, 1191–1195;
- 3bA. Reinhardt, Y. Yang, H. Claus, C. L. Pereira, A. D. Cox, U. Vogel, C. Anish, P. H. Seeberger, Chem. Biol. 2015, 22, 38–49;
- 3cL. Kong, B. Vijayakrishnan, M. Kowarik, J. Park, A. N. Zakharova, L. Neiwert, A. Faridmoayer, B. G. Davis, Nat. Chem. 2016, 8, 242–249.
- 4
- 4aX. Cao, X. Du, H. Jiao, Q. An, R. Chen, P. Fang, J. Wang, B. Yu, Acta Pharm. Sin. B 2022, 12, 3783–3821;
- 4bX. Zhang, H. Liu, L. Lin, W. Yao, J. Zhao, M. Wu, Z. Li, Angew. Chem. Int. Ed. 2018, 57, 12880–12885;
- 4cX. Zhang, W. Yao, X. Xu, H. Sun, J. Zhao, X. Meng, M. Wu, Z. Li, Chem. Eur. J. 2018, 24, 1694–1700;
- 4dX. Zhang, H. Liu, W. Yao, X. Meng, Z. Li, J. Org. Chem. 2019, 84, 7418–7425.
- 5
- 5aC. L. Bennett, M. C. Galan, Chem. Rev. 2018, 118, 7931–7985;
- 5bS. Meng, W. Zhong, W. Yao, Z. Li, Org. Lett. 2020, 22, 2981–2986.
- 6For reviews, see:
- 6aJ. Hansson, S. Oscarson, Curr. Org. Chem. 2000, 4, 535–546;
- 6bS. Oscarson, Carbohydr. Chem. 2012, 45, 40–60;
10.1039/9781849734769-00040 Google Scholar
- 6cT. K. Pradhan, K.-K. T. Mong, Isr. J. Chem. 2015, 55, 285–296;
- 6dP. Kosma, Tetrahedron Lett. 2016, 57, 2133–2142;
- 6eP. Kosma, Carbohydr. Chem. 2017, 42, 116–164;
- 6fT. K. Pradhan, Eur. J. Org. Chem. 2023, 26, e202300146.
- 7
- 7aS. Hashimoto, M. Hayashi, R. Noyori, Tetrahedron Lett. 1984, 25, 1379–1382;
- 7bM. Imoto, N. Kusunose, Y. Matsuura, S. Kusumoto, T. Shiba, Tetrahedron Lett. 1987, 28, 6277–6280;
- 7cM. Orbe, K. Luthman, T. Wåglund, A. Claesson, I. Csöregh, Carbohydr. Res. 1991, 211, 492763;
- 7dG.-J. Boons, F. L. van Delft, P. A. M. van der Klein, G. A. van der Marel, J. H. van Boom, Tetrahedron 1992, 48, 885–904;
- 7eH. Yoshizaki, N. Fukuda, K. Sato, M. Oikawa, K. Fukase, Y. Suda, S. Kusumoto, Angew. Chem. Int. Ed. 2001, 40, 1475–1480;
10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 7fY. Fujimoto, M. Iwata, N. Imakita, A. Shimoyama, Y. Suda, S. Kusumoto, K. Fukase, Tetrahedron Lett. 2007, 48, 6577–6581;
- 7gK. Mannerstedt, K. Ekelçf, S. Oscarson, Carbohydr. Res. 2007, 342, 631–637;
- 7hY. Zhang, J. Gaekwad, M. A. Wolfert, G.-J. Boons, Chem. Eur. J. 2008, 14, 558–569;
- 7iT. Ichiyanagi, M. Fukunaga, R. Tagashira, S. Hayashi, M. Nanjo, R. Yamasaki, Tetrahedron 2011, 67, 5964–5971;
- 7jA. Shimoyama, Y. Fujimoto, K. Fukase, Synlett 2011, 2359–2362;
- 7kA. Shimoyama, A. Saeki, N. Tanimura, H. Tsutsui, K. Miyake, Y. Suda, Y. Fujimoto, K. Fukase, Chem. Eur. J. 2011, 17, 14464–14474;
- 7lT. J. Boltje, W. Zhong, J. Park, M. A. Wolfert, W. Chen, G.-J. Boons, J. Am. Chem. Soc. 2012, 134, 14255–14262;
- 7mR. Yi, A. Ogaki, M. Fukunaga, H. Nakajima, T. Ichiyanagi, Tetrahedron 2014, 70, 3675–3682;
- 7nE. Mancuso, C. Romanò, N. Trattnig, P. Gritsch, P. Kosma, M. H. Clausen, Chem. Eur. J. 2021, 27, 7099–7102;
- 7oT. Ichiyanagi, H. Narimoto, N. Ohtani, Tetrahedron 2021, 96, 132397.
- 8
- 8aJ.-S. Huang, W. Huang, X. Meng, X. Wang, P.-C. Gao, J.-S. Yang, Angew. Chem. Int. Ed. 2015, 54, 10894–10898;
- 8bX.-Y. Zhou, P. Yang, S. Luo, J.-S. Yang, Chem. Asian J. 2019, 14, 454–461.
- 9
- 9aS. Hamajima, N. Komura, H.-N. Tanaka, A. Imamura, H. Ishida, H. Noguchi, T. Ichiyanagi, H. Ando, Org. Lett. 2022, 24, 8672–8676;
- 9bS. Hamajima, N. Komura, H.-N. Tanaka, A. Imamura, H. Ishida, H. Noguchi, T. Ichiyanagi, H. Ando, Molecules 2023, 28, 102.
- 10
- 10aX. Mi, Q. Lou, W. Fan, L. Zhuang, Y. Yang, Carbohydr. Res. 2017, 448, 161–165;
- 10bQ. Lou, Q. Hua, L. Zhang, Y. Yang, Org. Lett. 2020, 22, 981–985.
- 11J. Zhang, X. Gao, S. Liu, Z. Geng, L. Chang, Y. Liu, Q. Ma, G. Xing, G. Liu, D. Fang, Org. Lett. 2023, 25, 4150–4155.
- 12W. Huang, Y.-Y. Zhou, X.-L. Pan, X.-Y. Zhou, J.-C. Lei, D.-M. Liu, Y. Chu, J.-S. Yang, J. Am. Chem. Soc. 2018, 140, 3574–3582.
- 13
- 13aR. R. Schmidt, A. Esswein, Angew. Chem. Int. Ed. 1988, 27, 1178–1180;
- 13bA. Esswein, H. Rembold, R. R. Schmidt, Carbohydr. Res. 1990, 200, 287–305.
- 14P. Ngoje, D. Crich, J. Am. Chem. Soc. 2020, 142, 7760–7764.
- 15
- 15aP. Kosma, H. Sekljic, G. J. Balint, J. Carbohydr. Chem. 1996, 15, 701–714;
- 15bB. Pokorny, P. Kosma, Chem. Eur. J. 2015, 21, 305–313;
- 15cB. Pokorny, P. Kosma, ChemistryOpen 2015, 4, 722–728;
- 15dB. Pokorny, P. Kosma, Org. Lett. 2015, 17, 110–113;
- 15eN. Wimmer, PhD thesis, University of Natural Resources and Life Sciences-Vienna, 2001.
- 16K. Ikeda, S. Akamatsu, K. Achiwa, Chem. Pharm. Bull. 1990, 38, 279–281.
- 17
- 17aM. Reiner, R. R. Schmidt, Tetrahedron: Asymmetry 2000, 11, 319–335;
- 17bK. K. T. Mong, T. K. Pradhan, C.-H. Chiu, W.-C. Hung, C.-J. Chen, Y.-F. Wang, Org. Chem. Front. 2020, 7, 2179–2186.
- 18
- 18aK. Ikeda, S. Akamatsu, K. Achiwa, Carbohydr. Res. 1989, 189, 99075;
- 18bK. Ekelöf, S. Oscarson, Carbohydr. Res. 1995, 278, 289–300;
- 18cH. Tanaka, D. Takahashi, T. Takahashi, Angew. Chem. Int. Ed. 2006, 45, 770–773;
- 18dY. Yang, C. E. Martin, P. H. Seeberger, Chem. Sci. 2012, 3, 896–899;
- 18eT. K. Pradhan, C. C. Lin, K.-K. T. Mong, Org. Lett. 2014, 16, 1474–1477.
- 19G.-J. Boons, A. V. Demchenco, Chem. Rev. 2000, 100, 4539–4565.
- 20
- 20aE. R. Palmacci, O. J. Plante, P. H. Seeberger, Eur. J. Org. Chem. 2002, 595–606;
- 20b“Solid-phase oligosaccharide synthesis using glycosyl phosphates”: W.-C. Haase, O. J. Plante, P. H. Seeberger, in Solid Support Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries (Ed.: P. H. Seeberger), John Wiley & Sons, Inc, New York, 2001, pp. 117–134;
- 20c“Orthogonally protected building blocks for automated glycan assembly”: F. Pfrengle, P. H. Seeberger in Protecting Groups (Ed.: S. Vidal), Wiley-VCH, Weinheim, 2018, pp. 451–471.
- 21E. Onobun, D. Crich, J. Org. Chem. 2020, 85, 16035–16042.
- 22
- 22aM. A. Ghalambor, E. M. Levine, E. C. Heath, J. Biol. Chem. 1966, 241, 3207–3215;
- 22bY. Feng, J. Dong, F. Xu, A. Liu, L. Wang, Q. Zhang, Y. Chai, Org. Lett. 2015, 17, 2388–2391.
- 23
- 23aN. Y. Kulikova, A. M. Shpirt, L. O. Kononov, Synthesis 2006, 24, 4113–4114;
- 23bW. Fan, Y. Chen, Q. Lou, L. Zhuang, Y. Yang, J. Org. Chem. 2018, 83, 6171–6177.
- 24M. Nakamura, K. Takeda, H. Takayanagi, N. Asai, N. Ibata, H. Ogura, Chem. Pharm. Bull. 1993, 41, 26–30.
- 25R. Arihara, K. Kakita, N. Suzuki, S. Nakamura, S. Hashimoto, J. Org. Chem. 2015, 80, 4259–4277.
- 26
- 26aY. Hu, K. Yu, L.-L. Shi, L. Liu, J.-J. Sui, D.-Y. Liu, B. Xiong, J.-S. Sun, J. Am. Chem. Soc. 2017, 139, 12736–12744;
- 26bZ. Hu, Y. Tang, B. Yu, J. Am. Chem. Soc. 2019, 141, 4806–4810;
- 26cH. S. Kim, E. Jang, H. I. Kim, M. H. Babu, J.-Y. Lee, S. K. Kim, J. Sim, Org. Lett. 2023, 25, 3471–3475.
- 27“Tin. Silicon and Related Reducing Agents”: C. Chatgilialoylu, in Radicals in Organic Synthesis (Ed.: P. Renaud, M. P. Sibi), Wiley-VCH, Weinheim, 2001, pp. 28–49.
- 28
- 28aG. O. Aspinall, M. A. Monteiro, H. Pang, Carbohydr. Res. 1995, 279, 245–264;
- 28bE. V. Vinogradov, B. O. Petersen, J. E. Thomas-Oates, J. Ø Duus, H. Brade, O. Holst, J. Biol. Chem. 1998, 273, 28122–28131;
- 28cE. V. Vinogradov, J. Ø Duus, H. Brade, O. Holst, Eur. J. Biochem. 2002, 269, 422–430;
- 28dS. Leone, A. Molinaro, E. Pessione, R. Mazzoli, C. Giunta, L. Sturiale, D. Garozzo, R. Lanzetta, M. Parrilli, Carbohydr. Res. 2006, 341, 582–590;
- 28eE. Fregolino, G. Fugazza, E. Galano, V. Gargiulo, P. Landini, R. Lanzetta, B. Lindner, L. Pagani, M. Parrilli, O. Holst, C. De Castro, Eur. J. Org. Chem. 2010, 1345–1352.
- 29H. Paulsen, Angew. Chem. Int. Ed. 1982, 21, 155–173.
- 30J. Lodowska, D. Wolny, L. Węglarz, Can. J. Microbiol. 2013, 59, 645–655.
- 31Y. Kakitsubata, R. Aramaki, K. Nishioka, M. Wakao, Y. Suda, Tetrahedron Lett. 2016, 57, 1154–1157.
- 32
- 32aM. E. Falagas, E. A. Karveli, I. I. Siempos, K. Z. Vardakas, Epidemiol. Infect. 2008, 136, 1009–1019;
- 32bD. M. Sengstock, R. Thyagarajan, J. Apalara, A. Mira, T. Chopra, K. S. Kaye, Clin. Infect. Dis. 2010, 50, 1611–1616;
- 32cL. Antunes, P. Visca, K. J. Towner, Pathog. Dis. 2014, 71, 292–301.
- 33C. Willyard, Nature 2017, 543, 15.
- 34
- 34aM. Van Looveren, H. Goossens, Clin. Microbiol. Infect. 2004, 10, 684–704;
- 34bF. S. Taccone, H. Rodriguez-Villalobos, D. De Backer, V. De Moor, J. Deviere, J.-L. Vincent, F. Jacobs, Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 257–260;
- 34cR. Valencia, L. A. Arroyo, M. Conde, J. M. Aldana, M.-J. Torres, F. Fernández-Cuenca, J. Garnacho-Montero, J. M. Cisneros, C. Ortiz, J. Pachón, J. Aznar, Infect. Control. Hosp. Epidemiol. 2009, 30, 257–263.
- 35X.-Y. Zhou, L.-X. Li, Z. Zhang, S.-C. Duan, Y.-W. Huang, Y.-Y. Luo, X.-D. Mu, Z.-W. Chen, Y. Qin, J. Hu, J. Yin, J.-S. Yang, Angew. Chem. Int. Ed. 2022, 61, e202204420.
- 36For synthesis of 4,5-disubstituted Kdo glycosides, see:
- 36aN. Trattnig, J.-B. Farcet, P. Gritsch, A. Christler, R. Pantophlet, P. Kosma, J. Org. Chem. 2017, 82, 12346–12358;
- 36bT. Li, M. A. Wolfert, N. Wei, R. Huizinga, B. C. Jacobs, G.-J. Boons, J. Am. Chem. Soc. 2020, 142, 19611–19621.
- 37
- 37aGaussian 09, revision D.01, Gaussian, Inc.: Wallingford (CT), 2013;
- 37bC. Kee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789;
- 37cC. Gonzalez, H. B. Schlegel, J. Phys. Chem. 1990, 94, 5523–5527;
- 37dV. Barone, M. Cossi, J. Tomasi, J. Phys. Chem. 1997, 107, 3210–3221;
- 37eJ. Tomasi, B. Mennucci, R. Cammi, Chem. Rev. 2005, 105, 2999–3093;
- 37fA. Dan, M. Lergenmüller, M. Amano, Y. Nakahara, T. Ogawa, Y. Ito, Chem. Eur. J. 1998, 4, 2182–2190;
10.1002/(SICI)1521-3765(19981102)4:11<2182::AID-CHEM2182>3.0.CO;2-U CAS Web of Science® Google Scholar
- 37gJ. Liu, Y. Liu, Org. Lett. 2012, 14, 4742–4745.