Anion-(π)n-π Catalytic Micelles
Graphical Abstract
Anion-(π)n-π catalytic micelles are introduced to expand the collection of systems catalysts compatible with anion-π catalysis with a rich topic of general importance. They are of interest to realize anion-π catalysis, anion-(π)n-π catalysis and anion-(π)n-π autocatalysis in water. Classics in supramolecular chemistry are installed to control substrate binding and positioning within the confined micellar space for access to emergent properties.
Abstract
Anion-π catalysis operates by stabilizing anionic transition states on π-acidic aromatic surfaces. In anion-(π)n-π catalysis, π stacks add polarizability to strengthen interactions. In search of synthetic methods to extend π stacks beyond the limits of foldamers, the self-assembly of micelles from amphiphilic naphthalenediimides (NDIs) is introduced. To interface substrates and catalysts, charge-transfer complexes with dialkoxynaphthalenes (DANs), a classic in supramolecular chemistry, are installed. In π-stacked micelles, the rates of bioinspired ether cyclizations exceed rates on monomers in organic solvents by far. This is particularly impressive considering that anion-π catalysis in water has been elusive so far. Increasing rates with increasing π acidity of the micelles evince operational anion-(π)n-π catalysis. At maximal π acidity, autocatalytic behavior emerges. Dependence on position and order in confined micellar space promises access to emergent properties. Anion-(π)n-π catalytic micelles in water thus expand supramolecular systems catalysis accessible with anion-π interactions with an inspiring topic of general interest and great perspectives.
How strong are anion-π interactions? This is a frequently asked question concerning anion-π catalysis, that is the stabilization of anionic transition states on π-acidic surfaces (Figure 1a).1-5 The question arises from the intuitive perception of aromatic surfaces as rich in electrons and thus attracting cations rather than anions.6 The response is as for cation-π and other conventional noncovalent interactions: It depends. The π-acidic counterpart of benzene 1, that is hexafluorobenzene 2, has a quadrupole moment Qzz perpendicular to the aromatic plane that is similar in magnitude but opposite in sign (Figure 1c).1 In agreement with theory, ion binding energies for both 1 and 2 are similar.7, 8 For anion-π catalysis,1 hexafluorobenzene 2 holds thus as little promise as a simple benzene 1 for conventional cation-π catalysis.9
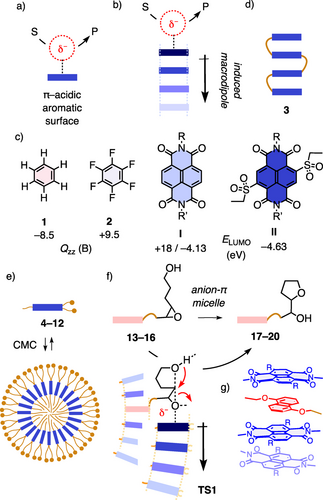
Definition of a) anion-π and b) anion-(π)n-π catalysis (S, substrate; P, product; red circle, transition state), with c) π-basic 1 compared to π-acidic 2, I and II (Qzz, quadrupole moment; B, Buckinghams; ELUMO, energy of LUMO; eV, electron volts) and d) foldamer 3. e) Anion-π catalytic micelle self-assembled from NDI amphiphiles 4–12 above their critical micelle concentration (CMC) in neutral water, with f) notional transition state TS1 for the cyclization of substrates 13–16, products 17–20, interfaced by g) NDI-DAN CT stacks (structures, see Figure 2).
Electron withdrawing substituents to increase Qzz and correspondingly decrease LUMO energies ELUMO offer one possibility to strengthen anion-π interactions (Figure 1c).1 For instance, the acidity of malonates covalently placed on top of an NDI I plane increases by ΔpKa=−1.8.10 Withdrawing substituents in the core up to sulfones II afford ΔpKa=−5.5, which corresponds roughly to the deprotonation of arginines in neutral water, considered impossible in biology.10 However, excessive electron deficiency also destabilizes the π system. To access really strong anion-π interactions for catalysis, transiently induced rather than permanent intrinsic anion-π interactions are thus overall more promising.1
Theory has predicted early on that anion-π interactions increase strongly on face-to-face stacks of π acids because polarization along the stacks produces supportive macrodipoles (Figure 1b).11 The power of such anion-(π)n-π interactions for anion-π catalysis has been confirmed with π-stacked foldamers 3 (Figure 1d).12 However, the synthesis of NDI foldamers is too demanding, realized examples never exceeded tetramers, and the inclusion of NDI core substitution is far beyond reach.12 Here, we explore the spontaneous self-assembly of NDI amphiphiles such as 4–12 into NDI micelles as supramolecular self-assembly strategy to access anion-(π)n-π catalysis on longer stacks beyond the limitations of covalent synthesis, including also core-substituted NDIs up to sulfone II (Figure 1c–g). Anion-(π)n-π catalytic micelles were also of interest to probe anion-π catalysis in water, usually13 avoided as solvent because of competing strong interactions.1
Catalysis in micelles has been realized in many variations.14-18 Attractive characteristics are easy access, mild conditions, high local concentrations, and enhanced interactions due to the absence of competing solvents. NDIs have been engineered extensively into micelles and higher order assemblies.19-24 Pioneering examples for catalysis in NDI micelles include Suzuki–Miyaura couplings,21, 22 an early aldolization catalyzed by an NDI nanotube with tethered prolines,23 and bisphenol A photodegradation in homologous PDI micelles,19 all possibly benefitting from anion-π interactions, although this possibility was neither mentioned nor explored. An excellent recent study reports anion-π interactions in monolayers of NDI amphiphiles at the air/water interface.25
The delocalized yet directional nature of anion-π interactions suggests that anion-π catalysis would be most suitable for reactions that involve electron displacement over long distances in coupled transition states. With the stabilization of multiple carbocation stabilization over π-basic arrays during terpene and steroid cyclization, lessons from nature support this expectation by analogy with canonical cation-π biocatalysis.9 Epoxide-opening polyether cyclizations, classics in chemistry and biology,26-31 have been identified recently as plausible anionic counterpart of steroid cyclization.32 Cascades up to tetramers have been realized with very simple small-molecule anion-π catalysts.32 However, anion-(π)n-π catalysis of epoxide-opening ether cyclizations has so far been realized neither in water nor in more complex systems. A first step toward anion-π systems catalysis in water, epoxide-opening ether cyclizations are explored in the following within anion-π catalytic micelles (Figure 1f). To interface epoxide substrates with anion-π catalytic NDI micelles, charge-transfer (CT) complex formation with DANs was envisioned (Figure 1g). The strongly colored NDI-DAN complexes are a classic in supramolecular chemistry, used, inter alia, in foldamers,33 dynamic libraries,34 rotaxanes,35 DNA architectures,36 sensors37, 38 and ion channels.38, 39 DAN interfacers should thus intercalate into NDI stacks in micelles and offer tethered substrates to a neighboring catalytic π-stack for conversion, as outlined in the notional transition state TS1 (Figure 1f).
To elaborate on these expectations, NDI amphiphiles 4–12 composed of hydrophilic heads H, hydrophobic tails T and catalytic cores C were prepared (Figure 2, Schemes S1–S4). Single-headed amphiphiles 4–7 were insoluble in water (not shown). According to dynamic laser light scattering (DLS), double-headed40, 41 amphiphile 8 yielded micelles in neutral buffer with a critical micelle concentration CMC=2 mM and a diameter of 7 nm (Figure S1, Table S1). Also in agreement with the Israelachvili rule of cones, cylinders and inverted cones,40, 41 replacement of the branched T2 by the longer and linear T1 in 9 lowered the CMC to 500 μM, while increasing the diameter slightly to 8.6 nm (Figure S2, Table S1). Double-headed amphiphiles 10 with sulfide donors in the core C3 were poorly soluble in water. However, oxidation to sulfoxides 11 gave micelles with low CMC=200 μM at constant diameter of 8.8 nm (Figures S4–S7, Table S1). Further oxidation to sulfones 12 lowered the CMC to record 100 μM, while the diameter increased to 9.2 nm (Figure S8, Table S1). At 30 °C, the diameter increased to 9.9 nm. Upon addition of 20 mol% substrate 13, micelles 12 swelled by 24 % to a diameter of 12.3 nm (Figure 3b). In agreement with DLS, transmission electron microscopy images of NDI micelles 11-D gave spherical particles with a diameter of 7–9 nm (Figure 3a).
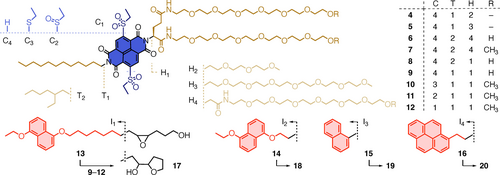
Structure of catalytic NDI amphiphiles 4–12 and substrates 13–17 with DAN interfacers and controls. H=head, T=tail, C=core, I=interfacer.
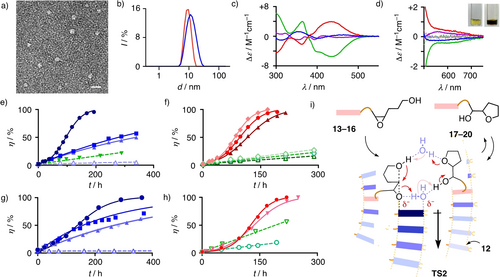
a) Transmission electron microscopy image of NDI micelles 11 (diastereoisomer D, 1 mM in phosphate buffer (10 mM Na2HPO4, pH=7.0, 100 mM NaCl) with 20 % of 13, scale bar=20 nm). b) Dynamic laser light scattering (DLS) of NDI micelles 12 without (red) and with (blue) 20 mol% substrate 13 (75 mM in phosphate buffer, at 30 °C). c) Circular dichroism (CD) spectra of diastereomers of micellar 11 (1 mM, A: blue, B: red, C: green, D: purple) in phosphate buffer. d) CD spectra of diastereomers of micellar NDI 11 (70 mM, A: blue, B: red, C: green, D: purple; the same in dotted lines: without 13) with 20 mol% DAN 13 in phosphate buffer (Inset: Solution of micellar 11 in buffer without (left) and with (right) 20 mol% 13). e) Conversion of substrate 13 (15 mM) with time in the presence of 75 mM monomeric NDI 9 in THF (light blue ▵) and micellar NDIs 9 (light blue ▴), 11 (blue ▪) and 12 (dark blue •) and Triton X-100 (green ▾) in phosphate buffer at 30 °C. f) Conversion of 7.5 mM (light diamonds), 15 mM (medium circles) and 25 mM (dark triangles) substrate 13 with time in the presence of micellar NDI 12 (red, filled) and Triton X-100 (green, open) in phosphate buffer at 30 °C. g) Conversion of substrate 14 (15 mM) with time in the presence of 75 mM monomeric NDI 9 in THF (light blue ▵) and micellar NDIs 9 (light blue ▴), 10 (lightest blue ⧫), 11 (blue ▪) and 12 (dark blue •) in phosphate buffer at 25 °C. h) Conversion of substrates 13 (circles) and 14 (triangles, both 15 mM) with time in the presence of 75 mM micellar NDI 12 (red, filled) and Triton X-100 (green, open) in phosphate buffer at 25 °C. i) Notional transition state TS2 for the autocatalytic conversion of 13–16 within anion-(π)n-π catalytic micelles 12. In e)-h), solid lines represent the best fit to the autocatalysis equation (Eq. S3) and the dashed ones to straight lines.
The four diastereomers A−D of sulfoxide amphiphiles 11 were separated by chiral HPLC to >82 % enantiopurity and labeled alphabetically according to their retention time (Scheme S5, Figure S9). Similar to NDI monomers, the circular dichroism (CD) spectra of their micelles showed the characteristic signatures of anti (A, D) and syn (B, C) sulfoxides, the latter diastereoisomers (B, C) characterized by strong, red-shifted CD Cotton effects (Figures 3c, S10).42, 43 Nearly mirror-imaged spectra for the anti and syn pairs AD and BC demonstrated the negligible influence of an additional stereogenic center in the glutamine headgroup on the chiroptical properties of the four diastereomers of 11. The addition of substrate 13 to micelles 11 produced the characteristic color of NDI-DAN CT complexes (Figure 3d, inset). In the enantioenriched NDI micelles 11, the CT complexation of DAN substrate 13 produced very weak CD signals at the expected long wavelength (Figure 3d).
To investigate ether cyclizations in NDI micelles, substrates 13–16 with more (I1) and less (I2) hydrophobic DAN, naphthalene (I3) and pyrene (I4) interfacers In were synthesized (Figure 2, Schemes S6–S7). Their conversion within anion-π catalytic micelles was monitored by HPLC (Figures S11–S14). For the lead substrate 13, the reaction in micelles was clearly faster than with monomers in THF. With NDI 9, the transition from monomers in THF to micelles in neutral phosphate buffer afforded a monomer-to-micelle rate enhancement of catalysis cemtm=kmicelle/kmonomer=8.2 (Figure 3e, ▴>▵). Among micellar NDIs 9, 11 and 12, catalytic activity increased with their π acidity (Figure 3e, •>▪>▴). This trend is the signature of operational anion-π catalysis.1 It thus implied that increasing transition-state stabilization by stronger anion-π interactions exceeds increasing ground-state stabilization by stronger charge-transfer complexes between substrate and supramolecular catalyst.44 Even the weakest anion-π catalytic micelle 9 was more active than Trition X-100, which forms active catalytic micelles (Figure 3e, ▴>▾).
Only within most π-acidic catalytic micelle 12, the conversion of substrate 13 showed autocatalytic kinetics (Figure 3e, •). Autocatalysis of polyether cascade cyclizations on π-acidic surfaces has been previously identified as a unique emergent property of anion-π catalysts.32, 45, 46 With monomeric NDI 12, the rate enhancement from autocatalysis compared to the initial rate of anion-π catalysts without product at the beginning of the reaction was aeo=kac(monomer)/kc(monomer)=320 (Table 1, entry 2). With micellar NDI 12, the rate enhancement of autocatalysis increased to ae=kac(micelle)/kc(micelle)=1600 (Table 1, entry 1). This calculated to an autocatalysis enhancement from monomer to micelle of aemtm=ae/aeo=5.0 (Table 1, entry 1). This increase was intriguing because the transition from monomers to micelles in reality is a transition from THF to water, in which anion-π catalysis should not occur. Anion-π autocatalysis in particular is best in apolar solvents with two molecules of water sandwiched between substrate and product like in a reversed mini-micelle (Figure 3i).47 Best activity with tethers that are both long and hydrophobic implied that anion-(π)n-π autocatalysis occurs deep inside the micelles, with the respective interfacers intercalating into nearby stacks with tethers long enough to place substrate, product and two molecules of water on top of the same polarizable π-stack, as outlined in TS2 (Figure 3i).
entry |
conditions[a] |
S[b] |
c [mM][c] |
ae[d] |
aemtm [e] |
|---|---|---|---|---|---|
1 |
micelle |
13 |
15 |
1600 |
5.0 |
2 |
monomer |
13 |
15 |
320 |
1.0 |
3 |
micelle |
13 |
7.5 |
1300 |
4.1 |
4 |
micelle |
13 |
25 |
1100 |
3.5 |
5 |
micelle |
14 |
15 |
470 |
1.5 |
6 |
micelle |
15 |
15 |
330 |
1.0 |
7 |
micelle |
16 |
15 |
800 |
2.5 |
- [a] Micelles: 75 mM NDI micelles 12 in phosphate buffer (10 mM Na2HPO4, pH=7.0, 100 mM NaCl) at 30 °C. Monomers: 75 mM NDI micelles 12 in THF at 30 °C. [b] Substrates, Figure 2. [c] Substrate concentration. [d] Rate enhancement for autocatalysis, ae=kac/kc, the autocatalytic rate constant divided by catalytic rate constant of a given reaction. [e] Rate enhancement for anion-(π)n-π autocatalysis ae in micelles divided by anion-π autocatalysis ae0 of 13 on monomers of 12 (entry 2).
This interpretation was supported by a drop of the record aemtm=5.0 for 13 to aemtm=1.5 for the more hydrophilic substrate 14 with unchanged DAN interfacers (Table 1, entry 5). At roughly constant hydrophobicity, a 50 % lower aemtm=2.5 for 16 with a pyrene supported the NDI-DAN CT classics (Figure 3d) as ideal for interfacing (Table 1, entry 1, 7). A 33 % lower aemtm=1.0 for 15 with a naphthalene as interfacer compared to aemtm=1.5 for DAN 14 supported this conclusion on the level of more hydrophilic, overall less convincing interfacers (Table 1, entry 5, 6).
The importance of micellar organization was confirmed by faster overall conversion with decreasing concentration of the best substrate 13 at constant concentration of the best NDI micelles 12 (Figure 3f, red, ⧫>•>▴). The same trend with the much weaker catalysis in Triton X-100 micelles suggested that decreasing micellar organization rather than substrate inhibition accounts for this inversion of conventional substrate dependence (Figure 3f, green, ◊>○>▵). In contrast to overall conversion, the isolate contributions from autocatalysis were with aemtm=5.0 highest at intermediate substrate concentration (Table 1, entry 1, 3, 4). Opposing contributions from increasing organization of both TS1 (high kc) and TS2 (high kac) but decreasing relative concentration of TS2 (high kac) with substrate dilution were likely to account for this small but interesting phenomenon.
The emerging importance of micellar organization was most visible in the 3.3-fold decrease of autocatalysis within the best NDI micelles 12 with decreasing hydrophobicity from substrate 13 to 14 (Figure 3h, • vs ▾, Table 1, entry 1, 5). A complementary 3.4-fold increase of catalysis, rather than decrease of autocatalysis, in Triton X-100 micelles demonstrated that the less hydrophobic substrate 14 stays near the surface of the Triton micelles to benefit from the known27, 48 contributions of water to catalyze ether cyclizations (Figure 3h, ○ vs ▿). In clear contrast, anion-(π)n-π autocatalysis as in TS2 excels with two molecules of water47 sandwiched between substrate and product on π surfaces in apolar environment with the more hydrophobic 13 deep within micelles but suffers in the presence of excess of water with the less hydrophobic 14 at the surface of NDI micelles 12. The depth of micelle penetration thus accounts for the maximal activity reached with substrate 13, sufficiently hydrophobic to interface for anion-(π)n-π autocatalysis in the best organized, least hydrated core of the most π-acidic NDI micelles 12.
The less hydrophobic substrate 14 reproduced the trends described for the deep-penetrating substrate 13 at overall lower activity. Namely, decreasing conversion rates with decreasing π acidity evinced operational anion-π catalysis (Figure 3g, •>▪>▴). A 6.3-faster conversion with even the least π-acidic micelles 9 compared to monomeric NDIs 9 confirmed the power of anion-(π)n-π catalysis (Figure 3g, ▴>▵). Rate enhancements with enantioenriched micelles 11 were almost independent of the sulfoxide stereochemistry (Figure S17, preliminary results with other substrates show large differences, opening perspectives also toward asymmetric catalysis).
The least π-acidic micelles 9 reproduced the trends described for the most π-acidic 12 at lower activity and without autocatalysis (Table S3). Catalytic rate enhancements compared to monomeric 9 decreased from 13 (cemtm=kmicelle/kmonomer=8.2) to 14 (6.3), 16 (5.0) and 15 (4.0, Figure S18). The ranking 13>14>16>15 for cemtm in 9 compared to 13>16>14>15 for aemtm in 12 confirmed that substrate hydrophobicity, that is deep micelle penetration, is more important for autocatalysis through the more sophisticated, fragile TS2, while CT interfacing is decisive for catalysis through the more robust TS1.
In summary, we report the design, synthesis and evaluation of anion-(π)n-π catalytic micelles. They are of interest to realize anion-(π)n-π catalysis and anion-(π)n-π autocatalysis in water, to extend π stacks beyond the limitations of covalent synthesis for efficient anion-(π)n-π catalysis, to explore classics in supramolecular chemistry, NDI-DAN complexes, as interfacers for substrate binding and positioning, and to prepare bioinspired polyether cyclizations for systems catalysis in water. The dependence of anion-(π)n-π autocatalysis on depth and order within the confined micellar space promises access to emergent properties that are otherwise beyond reach.46, 49
Supporting Information
Acknowledgments
We thank the NMR, MS and bioimaging platforms for services, the group of Prof. E. Bakker for assistance with DLS, and the University of Geneva, the National Centre of Competence in Research (NCCR) Molecular Systems Engineering (51NF40-182895) and the Swiss NSF for financial support (Excellence Grant 200020 204175; Swiss-ERC Advanced Grant TIMEUP, TMAG-2_209190). Open Access funding provided by Université de Genève.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.