Tetraazacoronenes and Their Dimers, Trimers and Tetramers
Graphical Abstract
Abstract
Tetraazacoronenes were synthesized from bay-functionalized tetraazaperylenes by Zr-mediated cyclization and four-fold Suzuki–Miyaura cross coupling. In the Zr-mediated approach, an η4-cyclobutadiene-zirconium(IV) complex was isolated as an intermediate to cyclobutene-annulated derivatives. Using bis(pinacolatoboryl)vinyltrimethylsilane as a C2 building block gave the tetraazacoronene target compound along with the condensed azacoronene dimer as well as higher oligomers. The series of extended azacoronenes show highly resolved UV/Vis absorption bands with increased extinction coefficients for the extended aromatic cores and fluorescence quantum yields of up to 80 % at 659 nm.
The synthesis of polycyclic aromatic hydrocarbons (PAHs) has been the focus of extensive research due to their applications in organic electronics.1 In the context of tuning their electronic properties, the isosteric [CH→N] substitution of polycyclic aromatics provides a method to vary the electronic structure of the material while maintaining the molecular shape and rigidity of the π-system.2 Whereas perylene-based dyes, such as perylene-3,4 : 9,10-bis(dicarboximides) (PDIs) have been among the most intensively studied chromophores in organic dye chemistry,3 a similar development of the structurally similar coronene was hampered by limited synthetic accessibility for a long time.4 More recently, the exploration of coronene dyes has experienced a renaissance based, inter alia, on the combination of the coronene core with the PDI-derived imido units.5 Examples for N-atom doping of coronene derivatives remain extremely rare,6, 7, 8 and only a single fully characterized tetraazacoronene has been reported.8b
Recently, we have begun to apply isosteric [CH→N] substitution to perylene chemistry giving synthetic access to various types of peri-functionalized tetraazaperylene derivatives.9-11 Herein we report two different strategies to laterally expand the azaperylene core in its bay-positions to give access to the corresponding tetraazacoronenes. On the basis of density-functional theory (DFT) simulations at the B3LYP/Def2-TZVPP-GD3(BJ)12-17 level of theory, the aza substitution should lower both the HOMO and LUMO energies about 1 eV (see Figure 1).
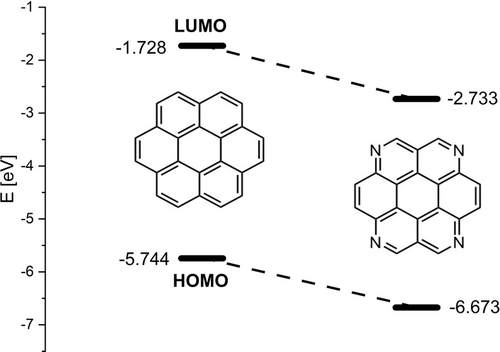
Calculated HOMO and LUMO energies of coronene and a model tetraazacoronene (B3LYP/Def2-TZVPP-GD3(BJ)).
In the first approach two adjacent sp hybridized carbon atoms are coupled via cyclization giving rise to a cyclobutene-annulated azacoronene. An alternative access to the tetraazacoronene core is achieved by fourfold Suzuki–Miyaura coupling of a >C=C< synthon which not only gives rise to the azacoronene parent compound but also to its two-, three- and four-fold condensed congeners.
The starting compound for the tetraazacoronene synthesis was the previously reported octaazaperopyrenedioxide (OAPPDO) derivative 1 (Scheme 1).11 Pd-catalyzed coupling of OAPPDO 1 with trimethyl((tributylstannyl)ethynyl)silane led to the fourfold alkynylated OAPPDO 2 (see SI). Compound 2 was then subjected to a zirconium mediated cyclization as established by D. Tilley and co-workers.18 The reactive ZrII species labelled as “Cp2Zr” were prepared in situ through reduction of Cp2ZrCl2 with n-butyllithium (“Negishi's reagent”),19 followed by addition of 2 as well as subsequent workup with acetic acid gave the trimethylsilyl (TMS) substituted doubly cyclobutene-annulated azacoronene 4 as the main reaction product. In 4 the cyclobutene moiety represents a strained four membered ring (bond angles of 85.4(2)–94.9(2)°) with an sp2−sp2 carbon bond length of 1.371(4) Å similar to coronene20 and a significantly elongated sp3−sp3 bond length of 1.614(4) Å (Figure 2).21 Compared to the parent coronene, the bond lengths of the aromatic core are similar except for the C−N bonds which are, as expected, slightly shorter.20
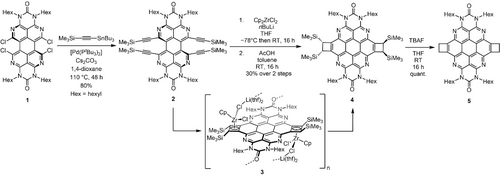
Synthesis of azacoronenes through Zr-mediated cyclization. Hex=n-hexyl.
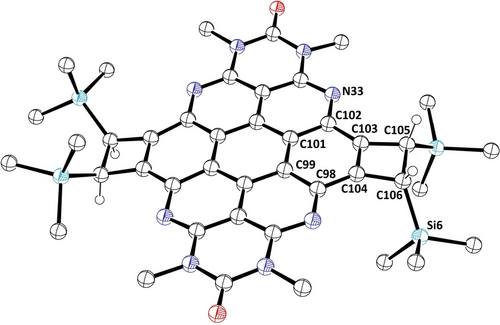
Molecular structure of azacoronene 4. Hydrogen atoms, disorder and hexyl substituents are omitted for clarity. Selected bond lengths in [Å]: C103−C105 1.532(4), C103−C104 1.371(4), C104−C106 1.521(4), C106−C105 1.614(4), C102−C101 1.414(4), C103−C102 1.420(4), N33−C102 1.378(4), C99−C98 1.412(4), C101−C99 1.421(4), C98−C104 1.425(4) and bond angles in [°]: C103−C105−C106 85.4(2), C104−C106−C105 85.5(2), C103−C104−C106 94.9(2), C104−C103−C105 94.2(2). Thermal ellipsoids at 50 % probability level.
Analysis of the electrochemical properties revealed that azaperylene 2 and azacoronene 4 possess two quasi reversible one electron oxidation steps (0.954/1.191 V for 2 and 1.039/1.349 V for 4 against a saturated calomel electrode, see SI).
The TMS groups of 4 could be removed with TBAF as a F− source forming the cyclobutene-annulated azacoronene 5. Its strained four-membered ring renders it a possible precursor for an electrocyclic ring-opening to afford the diene for consecutive Diels–Alder reactions. We have not observed reactions with substituted alkynes, however using N-ethylmaleimide as dienophile yielded the corresponding products. Separation of the stereoisomers remains subject of a future study.
The diastereoselective formation of compound 4 is already pre-determined by the relative orientation of the Zr-centers on both sides in the cyclization process. This was established by the isolation and structural characterization of the organometallic intermediate 3 by X-ray structure analysis. Its molecular structure, and particularly the coordination of the metal complex fragments, is depicted in Figure 3.21 The Zr fragment in 3 adopts a bent sandwich structure with two chlorido ligands, one of them bridging to a bis(thf-solvated) lithium cation. The latter connects one molecular unit with the urea oxygen atom of another azacoronene complex on each side which leads to the formation of a coordination polymer (Figure 3, top). The two π-coordinated ligands at Zr are the remaining Cp ligand and as well as a η4-bound cyclobutadiene moiety derived from the cyclization of the diynes in each bay area of the OAPPDO precursor.
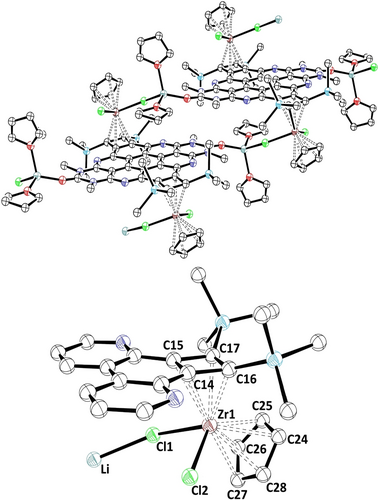
Molecular structure of coordination polymer 3. Hydrogen atoms and hexyl substituents are omitted for clarity. Selected bond lengths in [Å]: C14−C15 1.430(4), C14−C16 1.474(4), C16−C17 1.535(4), C17−C15 1.467(4), Zr1−C14 2.500(3), Zr1−C15 2.520(3), Zr1−C16 2.296(3), Zr1−C17 2.318(3), Li−Cl1 2.366(7) and bond angles in [°]: C17−C15−C14 92.1(2), C15−C14−C16 92.0(2), C14−C16−C17 87.7(2), C16−C17−C15 88.2(2). Thermal ellipsoids at 50 % probability level.
The four-membered π-ligand in 3 has distorted square geometry, with the C−C−C bond angles lying between 87.7(2)° and 92.1(2)°, while the C−C bond lengths range from 1.430(4) Å to 1.535(4) Å. The variation of the C−C distances is complementary to that of the Zr−C bonds (2.296(3)–2.520(3) Å) in that longer C−C bonds are associated with shorter Zr−C bonds.
We note that only Cp2-zirconacyclopentadienes have been isolated and characterized as intermediates of this type of cyclization reaction so far.18, 19b In general, η4-cyclobutadiene complexes of early transition metals are rare,22 the sole example for another zirconium cyclobutadiene species having been reported by Milsmann's group.23 Based on DFT modeling (B3LYP-GD3(BJ)/Def2-TZVPP)12-17 and localized orbital bonding analysis24, 25 the zirconium in intermediate 3 is best described as ZrIV (d0). A doubly reduced cyclobutadienyl moiety at both ends leads to an overall fourfold negative charge of the azacoronene-bis(cyclobutadienyl) unit (see SI). The long-ranging electrostatic repulsion between the zirconate units might be the main reason for the diastereoselectivity whereas short range steric effects (Pauli repulsion) are probably irrelevant. The HOMO and HOMO−1 π-orbitals resemble the two non-bonding orbitals of cyclobutadiene with only minor contributions of Zr d-orbitals, which is similar to the mononuclear complex previously studied by Milsmann.23
A second synthetic strategy for azacoronene derivatives of the OAPPDO precursor 1 without the annulated cyclobutene rings has been based on Suzuki–Miyaura cross coupling of (E)-(1,2-bis(pinacolatoboryl)vinyl)trimethylsilane as a vinylene building block for the bay-area of 1 and subsequent desilylation (Scheme 2). 1,2-Bis(pinacolatoboryl)alkenes are readily accessible by reaction of the corresponding alkynes with B2Pin2 in the presence of [Pt(PPh3)4]26 and are used as building blocks in the chemistry of functional PAHs,27 while the (E)-(1,2-bis(pinacolatoboryl)vinyl)trimethylsilane employed in this work has only been reported as a product of borylation catalysis.28 The azacoronene target 6 was obtained by this reaction sequence along the laterally condensed “dimeric” azacoronene 7 (after desilylation with TBAF) as major product when using 2.1 equivalents of the boronic ester.
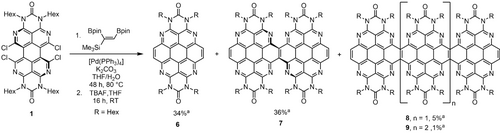
Synthesis of the azacoronene parent compound 6 and the corresponding condensed congeners 7, 8 and 9.[a] Combined yield using 1.5 eq. of bis(pinacolatoboryl)alkene. Hex=n-hexyl.
To obtain some mechanistic insights, model reactions of the C2 synthon under the same reaction conditions were performed (see SI) indicating that no Pd mediated isomerization to the corresponding Z diastereomer takes place. Analysis of the 1H- and 11B-NMR spectra revealed a desilylation of the synthon and a formation of other boron species. Reaction with bromobenzene showed no signs of a Heck type coupling competing against the Suzuki coupling. On this basis a tentative mechanistic proposal for the bay-annulation and competing oligomerization is displayed in Scheme 3. The first can be rationalized by a double Suzuki cross coupling reaction of the synthon similar to the mechanism described by Shimizu and co-workers.27a Concerning the oligomerization, after the first Suzuki coupling of the synthon a further azaaromatic system needs to be coupled to the vinyl moiety. This can proceed via desilylation or CH activation. Similar Pd catalyzed couplings with simpler systems are described in the literature.29, 30 A detailed investigation of the mechanism involving appropriate model compounds will be addressed in future studies.
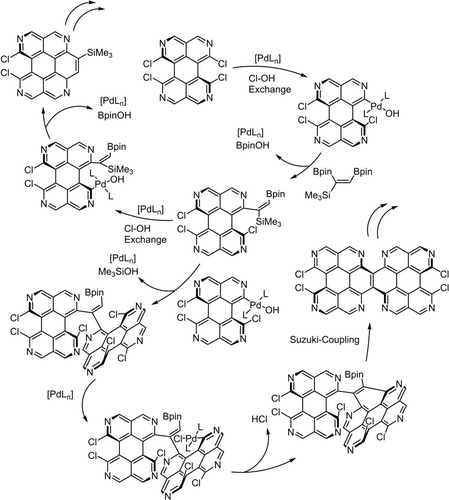
Cutout of a possible annulation and oligomerization mechanism depicting the key steps. For clarity, only one variant of the double Suzuki cross coupling and one variant of the desilylation/CH-activation/Suzuki coupling sequence is illustrated.
In order to assess whether higher oligomers of 6 were also formed during the reaction, we optimized the reaction conditions to promote further oligomerization (see SI). Using 1.5 equivalents of the boronic ester, not only 6 and 7 but also the corresponding three- and fourfold condensed expanded congeners 8 and 9 could be isolated and characterized. To the best of our knowledge, the azacoronene oligomers 7, 8 and 9 represent the first examples of heteroatom doped PAHs consisting of directly fused coronenes. In previous examples of lateral π-extension in PDI chemistry described by Nuckolls and co-workers, the PDI subunits are fused with >C=C< bridges.31 These nanoribbons were applied as materials for lithium batteries32 or in its twofold fused form as a polymer in solar cells.33 Further connection motifs are pyrene34 and naphthalene diimide units,35 or a direct fusion of two PDI molecules36 X-ray diffraction studies of 7 and 8 revealed that the individual planar coronene moieties are twisted with respect to each other by 32.998(15)° and 27.145(18)°, primarily due to steric pressure of the hexyl substituents (Figure 4 and Supporting Information for 7).21 In particular, the threefold extended compound 8 crystallizes in its meso and not a helically chiral conformation. Compared to doubly cyclobutene-annulated azacoronene 4, the bond lengths of the aromatic cores of 7 and 8 are similar except for the bond of the connecting carbon atoms being longer (1.439(3) vs. 1.371(4) Å).
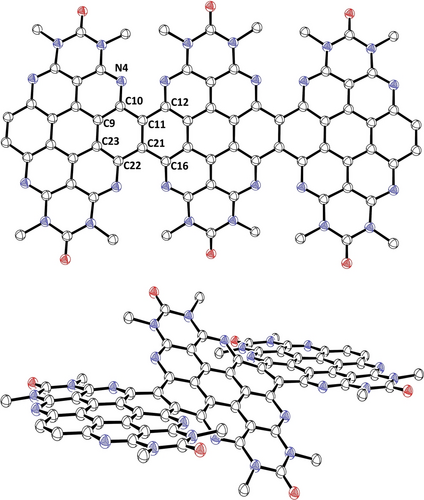
Molecular structure of the three-fold condensed azacoronene 8. Hydrogen atoms and hexyl substituents are omitted for clarity. Plane to plane twist angle Θ in [°]: 27.145(18); selected bond lengths in [Å]: N4−C10 1.370(3), C10−C11 1.452(3), C9−C10 1.397(3), C9−C23 1.409(3), C22−C23 1.399(3), C21−C22 1.463(3), C11−C21 1.439(3), C11−C12 1.455(3). Thermal ellipsoids at 50 % probability level.
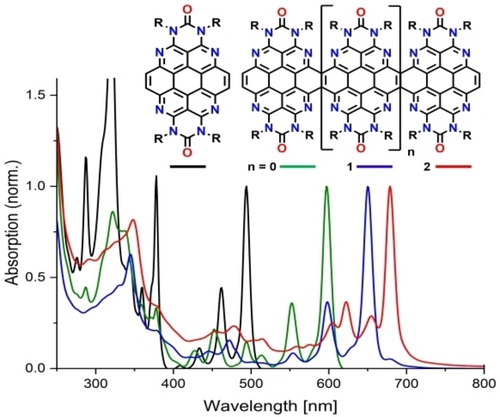
The UV/Vis absorption spectra of the azacoronene 6 and its fused extended congeners 7, 8, and 9 in solution are characterized by absorption bands in the visible spectral region with a distinct vibrational progression (Figure 5, see Supporting Information for a plot on a scale of molar absorption coefficient and for the spectra of azacoronenes 4 and 5). The effect of the π-extension on the bathochromic shift of the absorption maxima is most pronounced upon going from 6 to 7 (103 nm) and decays towards the higher oligomers (53 nm and 29 nm), while the molar extinction coefficients increase by one order of magnitude (Table 1). All compounds are highly fluorescent with the emission band being Stokes shifted by 150–200 cm−1 and the luminescence quantum yields of the oligomers higher than for the parent compound 6 (67–80 % versus 45 %).
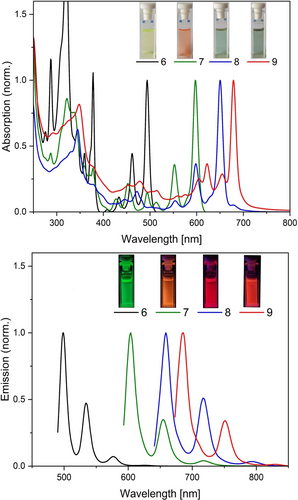
Normalized UV/Vis absorption and emission spectra of the azacoronene parent compound 6 and its condensed congeners 7, 8 and 9 in CHCl3.
|
λabs,max [nm] (log ϵ) |
λem,max [nm] (Φem)[a] |
Stokes-shift [cm−1] |
|---|---|---|---|
6 |
494 (4.48) |
499 (45 %) |
203 |
7 |
597 (4.99) |
604 (67 %) |
194 |
8 |
650 (5.26) |
659 (80 %) |
210 |
9 |
679 (5.05) |
686 (70 %) |
150 |
- [a] Fluorescence quantum yields measured with an Ulbricht sphere (E <0.1).
Based on TD-DFT modeling,12-17 the absorption maxima in the visible can be assigned to the first singlet transition (S0→S1) for all compounds and show a similar pattern of vibrational progression towards shorter wavelengths. Notably, there is another sharp absorption band in the long wave UV region observable in the spectrum of 6 which corresponds to the S0→S2 transition.
To analyze and quantify the local aromaticity of the azacoronenes and the effect of oligomerization on the aromatic ring current, the anisotropy of the induced current density (ACID) was analyzed (Figure 6).37 As for coronene itself,37b the density current vectors of 6 indicate a strong diatropic ring current running clockwise around the circulene periphery and a weak paratropic ring current in the central six membered ring. Fusion of further azacoronene moieties causes an expansion of the ring current leading to a delocalization around the whole molecule, shown here for 7 and similar to previously reported laterally benzo-fused coronenes38 (see Supporting Information for the ACID plots of the higher oligocoronenes and OAPPDO). It is therefore apparent that the lateral π-extension of the OAPPDO starting material, which itself displays the electronic characteristics of a tetraazaperylene (containing two fused diazanaphthalene units), leads to a full delocalization of the π-electrons. This is in stark contrast to a longitudinal π-extension in the form of the “rylene” series in which independent naphthalene units are fused with only partially coupled π-systems.39
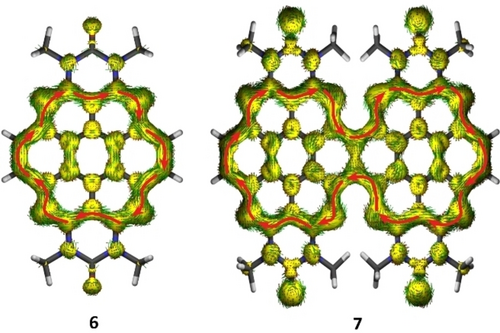
ACID of the π system of the azacoronene parent compound 6 and its two-fold condensed congener 7 at an isosurface value of 0.05.
The synthetic approach to a new class of azacoronene dyes has additionally provided a viable synthetic method to extended nanoscale azaaromatic systems with a high level of “N-doping”, the first examples of this series having been reported in this work. The relative ease of their isolation along with their pronounced fluorescence properties render this class of compounds an attractive target for further expansion towards (atomically precise) nanoribbons with adjustable optoelectronic properties and may find application as materials in lithium batteries as similar edge fused perylene diimide coronenes.
Acknowledgments
The authors acknowledge support by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through no. INST40/467-1FUGG (Justus cluster) and thank the DFG for funding within the SFB1249 (TP A2). We also thank Prof. Milan Kivala and Erik Misselwitz for help with measurement of the emission quantum yields. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.