Association of Radical Chemistry with LanD Flavoprotein Activity for C-Terminal Macrocyclization of a Ribosomal Peptide by Formation of an Unsaturated Thioether Residue
Graphical Abstract
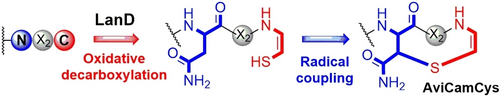
LanD flavoproteins catalyze oxidative decarboxylation of the C-terminal Cys residue of a peptide to produce a reactive enethiol. This enzyme activity can couple with radical S-adenosylmethionine chemistry to provide a new unsaturated thioether residue, S-[2-aminovinyl]-3-carbamoylcysteine, by conjugation of the enethiol with Cβ of the Asn residue in the C-terminal NxxC motif of a peptide for macrocyclization.
Abstract
LanD flavoproteins catalyze oxidative decarboxylation of the C-terminal Cys residue of a peptide to produce an enethiol. This enethiol is highly reactive and can be coupled with an upstream dehydroamino acid through Michael addition to form S-[2-aminovinyl](3-methyl)cysteine, an unsaturated thioether residue known to be characteristic of an array of C-terminally macrocyclized, ribosomally synthesized and posttranslationally modified peptides (RiPPs). Based on a two-stage bioinformatics mining of posttranslational modifications (PTMs) related to C-terminal Cys processing, we report herein that LanD activity can couple with radical S-adenosylmethionine chemistry to provide a new unsaturated thioether residue, S-[2-aminovinyl]-3-carbamoylcysteine, by conjugating the resultant enethiol with Cβ of the Asn residue in the C-terminal NxxC motif of a peptide for macrocyclization. This study furthers our understanding of the variety of PTMs involved in creating the structure diversity of macrocyclic RiPPs.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.