Bonding and Acidity of the Formal Hydride in the Prototypical Au9(PPh3)8H2+ Nanocluster
Graphical Abstract
Surface-bound hydrogen has been shown to improve chemical reactivity and selectivity in gold clusters. However, the chemical properties and reactivity of these hydrogen atoms is difficult to directly probe. We show for the prototypical cluster Au9(PPh3)8H2+ that the hydrogen bonds to the cluster in a manner somewhere between a covalent bond and a particle in a box, and that it is likely to behave as a moderate acid.
Abstract
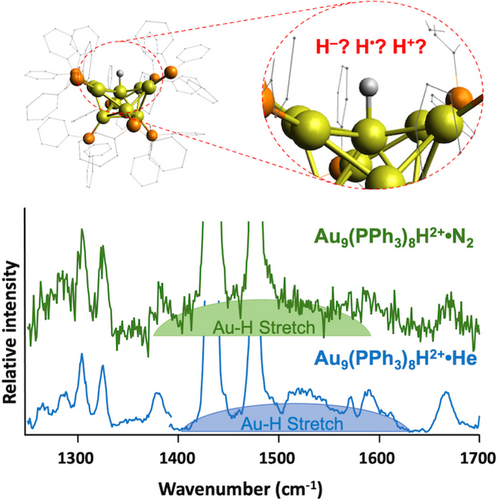
The role of hydrogen atoms as surface ligands on metal nanoclusters is of profound importance but remains difficult to directly study. While hydrogen atoms often appear to be incorporated formally as hydrides, evidence suggests that they donate electrons to the cluster's delocalized superatomic orbitals and may consequently behave as acidic protons that play key roles in synthetic or catalytic mechanisms. Here we directly test this assertion for the prototypical Au9(PPh3)8H2+ nanocluster, formed by addition of a hydride to the well-characterized Au9(PPh3)83+. Using gas-phase infrared spectroscopy, we were able to unambiguously isolate Au9(PPh3)8H2+ and Au9(PPh3)8D2+, revealing an Au−H stretching mode at 1528 cm−1 that shifts to 1038 cm−1 upon deuteration. This shift is greater than the maximum expected for a typical harmonic potential, suggesting a potential governing cluster-H bonding that has some square-well character consistent with the hydrogen nucleus behaving as a metal atom in the cluster core. Complexing this cluster with very weak bases reveals a redshift of 37 cm−1 in the Au−H vibration, consistent with those typically seen for moderately acidic groups in gas phase molecules and providing an estimate of the acidity of Au9(PPh3)8H2+, at least with regard to its surface reactivity.
Conflict of interest
The authors have no conflict of interest to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.