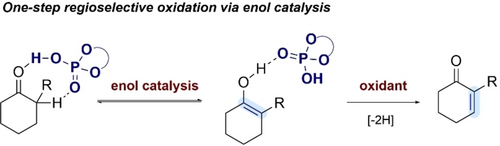
Direct Regioselective Dehydrogenation of α-Substituted Cyclic Ketones
Graphical Abstract
A highly regioselective, one-step dehydrogenation of α-substituted cyclic ketones in the presence of 2,3-dichlorobenzo-5,6-dicyano-1,4-benzoquinone is described. The reaction proceeds via phosphoric acid-based enol catalysis and provides access to several α-aryl and α-alkyl substituted α,β-unsaturated ketones.
Abstract
We disclose a highly regioselective, catalytic one-step dehydrogenation of α-substituted cyclic ketones in the presence of 2,3-dichlorobenzo-5,6-dicyano-1,4-benzoquinone (DDQ). The high regioselectivity originates from a phosphoric acid-catalyzed enolization, selectively affording the thermodynamically preferred enol, followed by the subsequent oxidation event. Our method provides reliable access to several α-aryl and α-alkyl substituted α,β-unsaturated ketones.
The dehydrogenation of carbonyls to the corresponding α,β-unsaturated pendants represents an often-encountered transformation within the synthesis of complex molecules and natural products.1 A general two-step strategy relies on the activation of ketones as either enolate or enol silane (Figure 1-A), wherein the deprotonation under thermodynamic or kinetic control dictates the regioselectivity of the subsequent oxidation event. The desaturation of enolates is often performed in the presence of chalcogen halides (Reich, Trost, Mukaiyama), whereas silyl enol ethers are frequently oxidized in the presence of Pd(OAc)2 (Saegusa-Ito).2-5 Aiming to avoid toxic chalcogen-derived oxidants or costly stoichiometric palladium-based oxidants, much effort has been devoted toward the development of less toxic, cheaper, and catalytic variants. Recently, Baran and co-workers developed an electrochemically driven desaturation of pre-formed enol surrogates.6 An early catalytic example has been described by Larock and co-workers, focusing on the Seagusa-Ito oxidation of silyl enol ethers employing catalytic amounts of Pd(OAc)2 in the presence of oxygen as terminal oxidant.7 Stahl et al. further improved the palladium-based oxidation strategy, enabling a direct oxidation of unactivated ketones using catalytic amounts of palladium-derived catalyst under an oxygen atmosphere (Figure 1-B),8 whereas Newhouse has demonstrated the oxidation of pre-formed kinetic enolates utilizing diethyl allyl phosphate as an oxidant.9 Recently, Kang and Qu demonstrated that the combination of FeCl3 with 1,10-phenanthroline represents a powerful catalytic system for TEMPO-mediated desaturations.10
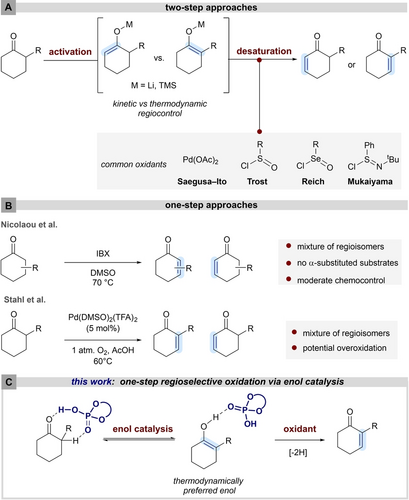
A) Two-step approach of unsaturation of cyclic α- substituted ketones. B) One-step approach. C) This work: one-step regioselective oxidation via enol catalysis.
A metal-free, one-step dehydrogenation of ketones has been reported by Nicolaou, employing hypervalent iodine reagents (Figure 1-B).11 Although these one-step strategies have significantly advanced desaturation methodologies and are often employed in total syntheses, common drawbacks still limit their general applicability. For instance, insufficient regiocontrol can lead to moderate yields and challenging separation requirements. Additionally, protecting groups may be needed for functional groups, which are prone to oxidations, sometimes limiting the methods to relatively unfunctionalized substrates. To the best of our knowledge only an enzymatic approach has achieved high levels of regioinduction, albeit in unsatisfactory conversions.12
We wondered if phosphoric acid based enol catalysis may be utilized to design a new approach that could solve some of the above issues. Enol catalysis combines the prerequisite of activating ketones in combination with high regiocontrol toward the thermodynamically more stable enol, as demonstrated in enantioselective Aldol-, α-amination-, α-hydroxylation-, Michael-, and aryloxylation reactions.13-18 Furthermore, Schmittel et al. have confirmed the lower oxidation potential of enols in comparison to the parent ketones.19, 20 We therefore anticipated that the combination of enol catalysis with a suitable oxidant may enable a novel entry point to develop a catalytic one-step ketone desaturation, avoiding the previously encountered regioselectivity issues (Figure 1-C).
An initial reaction condition screening was performed with 2-phenylcyclohexanone (1 a) as a model substrate in the presence of diphenyl phosphoric acid (DPP) as catalyst and several oxidants (see Supporting Information for further information). In particular 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), a widely utilized dehydrogenating agent, turned out to be a suitable oxidant for our envisioned enol catalysis mediated desaturation strategy.21-24 The desired enone 2 a along with the corresponding α-aryloxylated product 3 a were obtained from ketone 1 a (entry 1, Table 1), albeit in uncomplete conversion.17, 25 Notably, the reaction does not proceed without the phosphoric acid catalyst (entry 2), consistent with the envisioned phosphoric acid-mediated enolization as activation mode. After a thorough screening of reaction conditions, including the investigation of several solvents (entries 3–6), other additives, oxidants, and reaction temperatures (see Supporting Information for further information), the yield of the desired enone remained unsatisfactory and significant amounts of side product 3 a were still obtained. Attempts to transform side product 3 a in situ into the desired enone 2 a remained elusive. Interestingly, the application of commercially available enantiomerically enriched BINOL-derived phosphoric acids (CPA) had a notable effect on the product distribution, favoring the formation of the desired unsaturated enone 2 a (entries 7–9).
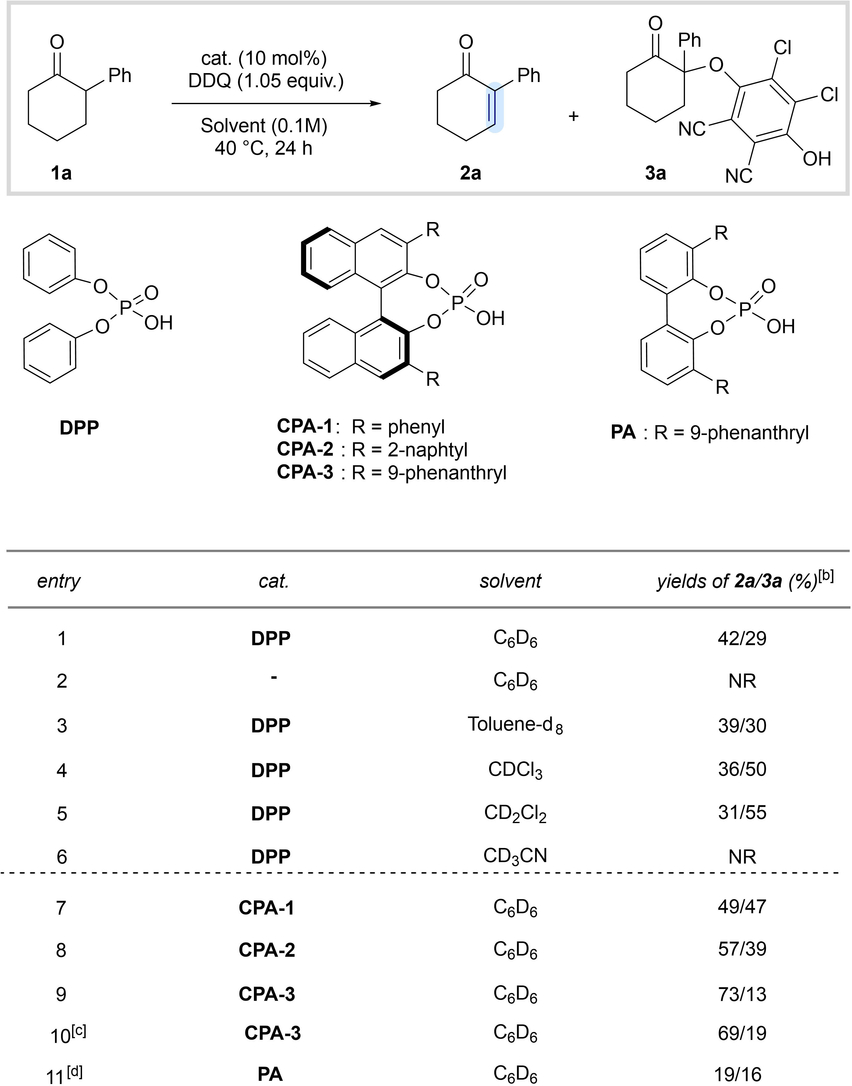
- [a] Reactions were performed on 0.05 mmol scale. [b] Yields were determined by 1H NMR using dibromomethane as internal standard. [c] Reaction was performed using racemic CPA-3. [d] Low catalyst solubility was observed.
Catalyst CPA-3, containing the 3,3'-(9-phenanthryl) substituent, was found to be a superior catalyst scaffold, either enantiomerically pure (entry 9) or as a racemate (entry 10). A phosphoric acid based on the biphenyl scaffold (PA) was also investigated, which however provided significantly lower yields owing to its low solubility (see Supporting Information for further information).
With commercially available phosphoric acid CPA-3 in hand, we further explored the scope and generality of our new desaturation method (Tables 2 and 3). α-Phenylcyclopentanone (2 b) and α-phenylcycloheptanone (2 c) smoothly underwent the desired oxidation, albeit in slightly reduced yield for the 7-membered cyclic substrate (Table 2, entry 2–3). The lower yield for the 7-membered cyclic substrate may be explained with higher energy requirement for the transition state within the enolization event causing diminished reactivity. α-Arylcyclohexanones with electron-deficient and electron-rich substituents are well suited, furnishing the desired products in good yields (entries 4–6). The methodology also tolerates structurally enlarged α-substituents as shown in entries 7 and 8. In addition, aliphatic α-substituents are well tolerated (Table 3), illustrated with enone 2 i (entry 1). Substituents, which are sensitive to oxidative conditions, such as benzylic substituents (entry 2) or dithioacetals (entry 5) reacted smoothly toward the desired enones 2 j and 2 m, respectively. Remarkably enone 2 l was obtained as a single regioisomer from 2,4-dimethylcyclohexanone (1 l) (entry 4).
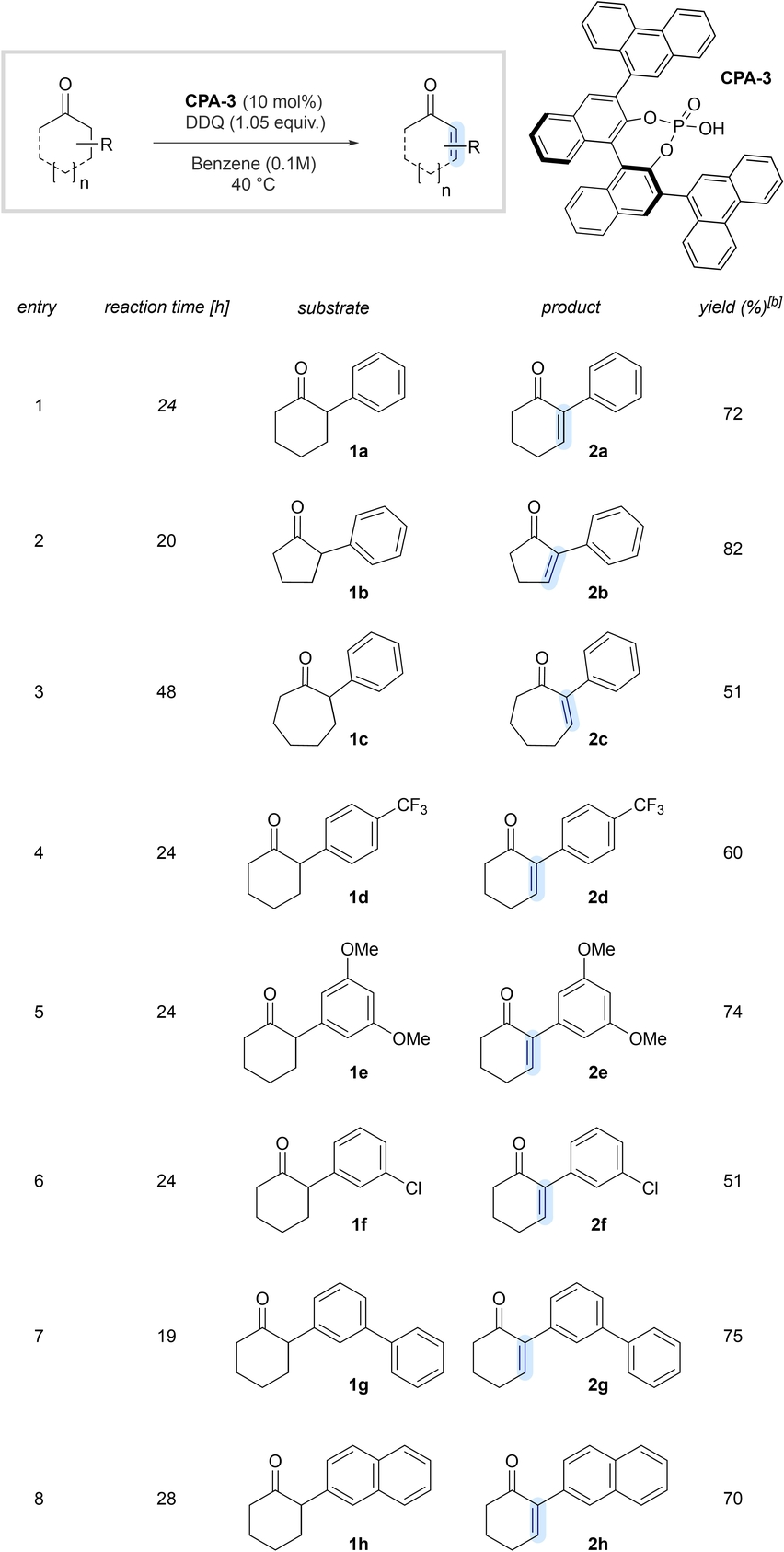
- [a] Reactions were performed on 0.5 mmol scale. [b] Isolated yields (r.r>20 : 1).
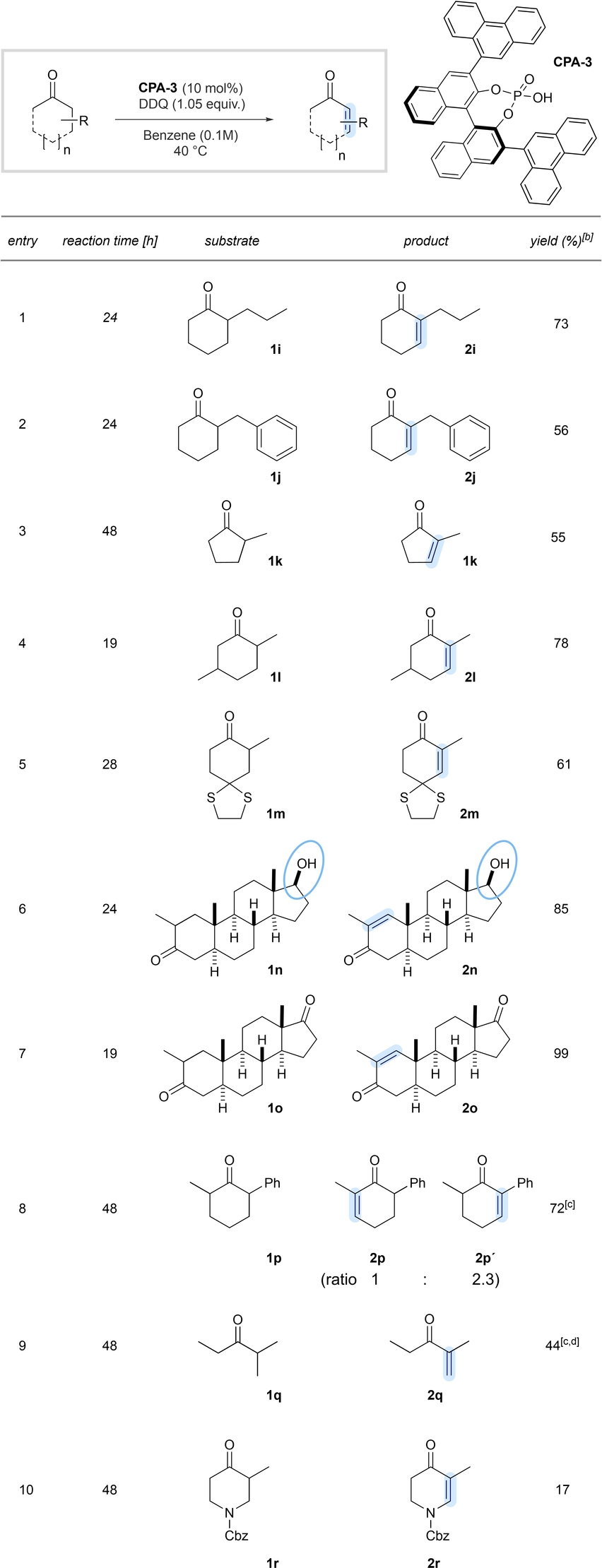
- [a] Reactions were performed on 0.5 mmol scale. [b] Isolated yields (r.r>20 : 1). [c] NMR yields. [d] Reaction was performed in toluene-d8 (0.1 M), 48 h at 60 °C.
We also elaborated α-substituted steroidal substrates. Notably, steroid 1 n, containing an unprotected secondary alcohol at the D ring, was selectively desaturated without any undesired alcohol oxidation (entry 6). Diketone 1 o underwent a selective dehydrogenation at the A-ring, having the α-methyl moiety attached to provide an excellent yield of the desired unsaturated diketone 2 o in 99 % yield (entry 7).26 2,6-substituted substrates, such as 2-phenyl-6-methylcyclohexanone 1 p afforded a mixture of two regioisomeric products, in which the enolization event is seemingly favoured at the α-phenyl moiety. An acyclic substrate 1 q also undergoes the desired desaturation event at the higher substituted position, albeit in reduced yields under modified reaction conditions. Substrates containing nitrogen substituents, as indicated with the Cbz-protected piperidinone 1 r, provided significantly reduced yields of the desired enone due to undesired side reactions.
To investigate the mechanism of our new oxidation reaction and to elucidate if the proposed enol catalysis pathway is indeed in operation, we conducted radical clock and NMR experiments. First, we subjected substrates 1 p and 1 q to our reaction conditions (Figure 2-A). If the reaction would proceed via a proton coupled electron transfer (PCET), as suggested for a related α-aryloxylation,13 the formation of an alpha-keto radical may lead to a fragmentation or cyclization reaction to give α-allylcyclohexanone or a spirocyclic product, respectively. Instead, only the corresponding unsaturated products were obtained in good overall yields, making a radical pathway less likely. Second, to address the question if DDQ-adducts such as ketone 3 a,21 are intermediates, NMR monitoring of the reaction with model substrate 1 a either with the optimal catalyst CPA-3 or with DPP (Figure 2-B) was performed. Clearly, adduct 3 a is not an intermediate but formed in a parallel reaction. The kinetic profile in the presence of both catalysts is in agreement with the experimental results and the NMR yields of product formation match with the isolated yields. Accumulation and follow-up decay of other intermediates, which may form within a polar reaction pathway were not detected. However, 31P NMR monitoring indicates slow decay of the phosphoric acids into unidentified species (see Supporting Information for further information). In view of these results, we suggest a reaction mechanism, in which the phosphoric acid catalyst templates a simultaneous proton and hydride shift mechanism akin to phosphoric acid catalyzed transfer hydrogenation by Hantzsch esters.27 The phenanthryl substituent could stabilize such a transition state by π-π interaction with DDQ.19 Indeed, DFT-optimization of a complex between CPA-3, the enol of 1 a, and DDQ at the B3LYP-D3/def2-SVP level of theory (Figure 3) is consistent with this model. Both reactants are closely arranged by the bifunctional CPA catalyst through two tight hydrogen bonds. The overall complex forms a sandwich-like structure, stabilizing DDQ by virtue of close π–π contacts to the 3,3′-phenanthryl groups of the BINOL as well as the electron-rich phenyl-substituted enol moiety (3.35 Å and 3.00 Å, respectively). A competing proton-coupled electron transfer (PCET) of in situ generated enol with DDQ may occur, which upon radical recombination explains the formation of the undesired aryloxylated side product.
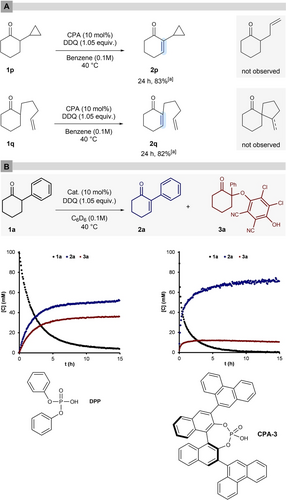
A) Evaluation of the substrates 1 q and 1 r in our transformation under standard conditions. B) 1H NMR reaction monitoring using CPA-3 and DPP.[a] Isolated yields (r.r.>20 : 1).

Plausible catalytic pathways. B3LYP-D3/def2-SVP-optimized structure of the tertiary complex between CPA-3, 1 a-enol and DDQ. R3PO-enol 1.59 Å, R3POH-DDQ 1.52 Å. π–π distances: phenanthryl-DDQ 3.35 Å, DDQ-enol/Ph 3.00 Å.
Clearly, alternative mechanisms should not be excluded at this point and we would like to refer to detailed studies from Mayr and co-workers on DDQ mediated oxidations.28
In summary, we report a one-step approach to selectively desaturate cyclic ketones, proceeding via phosphoric acid based enol-catalysis. The methodology selectively furnishes higher substituted α,β-unsaturated aryl- and alkyl- substituted enones in good yields without any observable overoxidation toward phenols. Functional groups, which are sensitive toward oxidative conditions, such as alcohols or dithioacetals, are well tolerated. We anticipate that the method reported here complements existing procedures and may find utility in chemical synthesis.
Acknowledgments
Generous support from the Deutsche Forschungsgemeinschaft (Leibniz Award to B.L. and Cluster of Excellence Ruhr Explores Solvation, RESOLV), and the European Research Council (ERC, European Union's Horizon 2020 research and innovation program “C−H Acids for Organic Synthesis, CHAOS” Advanced Grant Agreement No. 694228) is gratefully acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.