Synthesis of Cobalt-Tin and -Lead Tetrylidynes—Reactivity Study of the Triple Bond
Graphical Abstract
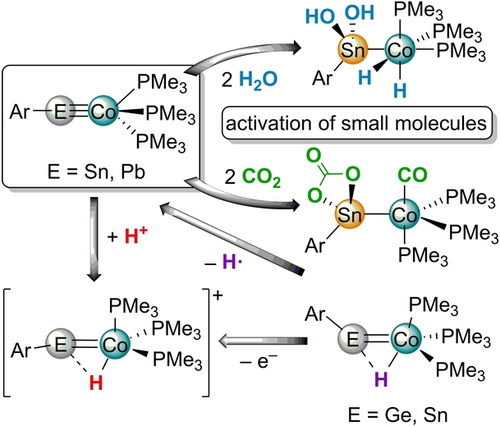
Tetrylidynes featuring a triple bond between cobalt and tin or lead have been synthesized via a substitution reaction between anionic cobalt complex [Co(PMe3)4]− and halides of tin and lead. The cobalt stannylidyne adds two equivalents of water to give a cobalt dihydride moiety and tin dihydroxide unit. In reaction with carbon dioxide the stannylidyne forms a cobalt carbonyl complex featuring a carbonate ligand coordinated at tin.
Abstract
Tetrylidynes [TbbSn≡Co(PMe3)3] (1 a) and [TbbPb≡Co(PMe3)3] (2) (Tbb=2,6-[CH(SiMe3)2]2-4-(t-Bu)C6H2) are accessed for the first time via a substitution reaction between [Na(OEt2)][Co(PMe3)4] and [Li(thf)2][TbbEBr2] (E=Sn, Pb). Following an alternative procedure the stannylidyne [Ar*Sn≡Co(PMe3)3] (1 b) was synthesized by hydrogen atom abstraction using AIBN from the paramagnetic hydride complex [Ar*SnH=Co(PMe3)3] (4) (AIBN=azobis(isobutyronitrile)). The stannylidyne 1 a adds two equivalents of water to yield the dihydroxide [TbbSn(OH)2CoH2(PMe3)3] (5). In reaction of the stannylidyne 1 a with CO2 a product of a redox reaction [TbbSn(CO3)Co(CO)(PMe3)3] (6) was isolated. Protonation of the tetrylidynes occurs at the cobalt atom to give the metalla-stanna vinyl cation [TbbSn=CoH(PMe3)3][BArF4] (7 a) [ArF=C6H3-3,5-(CF3)2]. The analogous germanium and tin cations [Ar*E=CoH(PMe3)3][BArF4] (E=Ge 9, Sn 7 b) (Ar*=C6H3(2,6-Trip)2, Trip=2,4,6-C6H2iPr3) were also obtained by oxidation of the paramagnetic complexes [Ar*EH=Co(PMe3)3] (E=Ge 3, Sn 4), which were synthesized by substitution of a PMe3 ligand of [Co(PMe3)4] by a hydridoylene (Ar*EH) unit.
Metal-ligand multiple bonds are of interest for an investigation of the bonding parameters and for reactivity studies. In the case of the higher homologues of carbynes exhibiting a metal-carbon triple bond a variety of examples of tetrylidyne complexes with element combinations between metals M=Nb,1 Cr,2 Mo,3 W,3c, 3e, 3g, 4 Mn,5 Re,6 Fe,1 Os,7 Rh,8 Ni,9 Pt1 and main group elements Si, Ge, Sn and Pb have been reported.10 Reactivity studies of tetrylidyne complexes have been reported in the case of silylidyne and germylidyne complexes. 2+2-cycloadditon reactions, CH-activation, addition of nucleophiles at the Si-atom and addition of alcohols and α,β-unsaturated ketones have been studied.7, 11 We became interested in the chemistry of tetrylidyne complexes because we explored a dehydrogenation reaction of dihydrido bridged rhodium-tin [(Ph3P)2Rh(μ-H)2SnAr*] and rhodium-lead [(Ph3P)2Rh(μ-H)2PbAr*] complexes to yield Rh≡Sn and Rh≡Pb tetrylidynes.8 Furthermore, these complexes show addition of one equivalent hydrogen in the lead case and two equivalents in the tin case. Remarkably, the hydrogen addition at the tin atom of [(Ph3P)2H2RhSnH2Ar*] is a reversible reaction at room temperature. In this publication we report on the synthesis of unprecedented cobalt tetrylidynes of tin and lead and reactivity studies of the cobalt stannylidyne with water, carbon dioxide and a Brønsted acid.
In a one-step procedure the cobalt tetrylidyne complexes (1 a, 2) were synthesized (Scheme 1) treating the anionic cobalt phosphine complex [Na(OEt2)][Co(PMe3)4] with the low valent organodibromostannate(II) [Li(thf)2][TbbSnBr2] and -plumbate(II) [Li(thf)2][TbbPbBr2] at 6 °C in benzene. Stannylidyne [TbbSn≡Co(PMe3)3] (1 a) was isolated as a blue crystalline substance in a yield of 51 % and the plumbylidyne [TbbPb≡Co(PMe3)3] (2) as reddish brown crystals in a yield of 47 % (Scheme 1). Interestingly, Power, Wolf and co-workers treated anionic [(COD)2Co]− with [(Ar'SnCl)2] to give a dimeric Co−Sn cluster compound [Ar'SnCo]2 (Ar’=C6H3-2,6(C6H3-2,6-iPr2)2).12
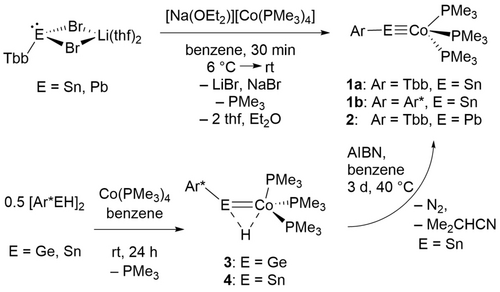
Formation of cobalt tetrylidynes 1 a, 1 b, 2 and synthesis of hydrides 3 and 4.
Following an alternative synthetic pathway, the stannylidyne 1 b was obtained by hydrogen atom abstraction from the paramagnetic hydride complex 4. Hydrides 3 and 4 were synthesized by phosphine substitution treating the cobalt complex [Co(PMe3)4] with low valent hydrides of germanium and tin (Scheme 1).13 In reaction with AIBN at 40 °C the paramagnetic complex 4 was converted via hydrogen atom abstraction into the stannylidyne complex 1 b. The germanium compound 3 was also treated with AIBN, but formation of a germylidyne complex was not observed.
Crystals of 1 a, 1 b and 2 were isolated from the synthetic mixtures and the molecular structures of 1 b and 2 are shown in Figure 1 (1 a see SI), while Table 1 lists selected interatomic distances and angles. The most striking features of the molecular structures of 1 a, 1 b and 2 are the near-linear Co−Sn−C1 [179.7(1)°] and Co−Pb−C1 [173.4(1)°] arrangements together with the shortest bond lengths between these elements Co−Sn [2.2804(3) Å] and Co−Pb [2.3315(3) Å] reported so far. Short Co−Sn distances have been reported for [Cp*Co−Sn{(CSiMe3)2}2]14 [2.386(2) Å] and for [(Ar*CoSn)2] [2.4071(6) Å] exhibiting partial Sn−Co double bond character.12 In the case of the Co−Pb bond only a few complexes have been reported showing a range of interatomic distances of 2.761(2) Å–2.6191(4) Å.15 The coordination sphere around the cobalt atoms in 1 and 2 can be described as a tetrahedral arrangement and is comparable with the homologous rhodium complexes.8 The electronic structures of the complexes were evaluated using DFT calculations (wB97X-def2-SVP/TZVP(Co,Sn,Pb)). The short Co≡E bond lengths and the large C1−E−Co angle were reproduced by these calculations (E = Sn, Pb). The HOMO, and HOMO-1 resembling the π-bonds are shown for the Co−Pb case in Figure 2.
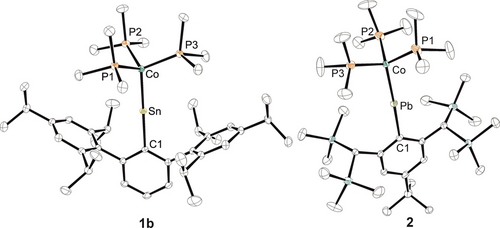
ORTEP of the molecular structures of 1 b and 2 (1 a see SI). Thermal ellipsoids are shown at 50 % probability level. Hydrogen atoms have been omitted.
|
1 a (E=Sn) |
1 b (E=Sn) |
2 (E=Pb) |
|---|---|---|---|
E−Co |
2.2804(3) |
2.2829(3) |
2.3315(3) |
C1−E |
2.171(2) |
2.188(2) |
2.249(2) |
C1−E−Co |
173.4(1) |
179.7(1) |
173.4(1) |
Co−P1 |
2.1481(8) |
2.1539(5) |
2.1490(7) |
Co−P2 |
2.1447(8) |
2.1590(6) |
2.1487(7) |
Co−P3 |
2.1492(8) |
2.1521(5) |
2.1464(7) |
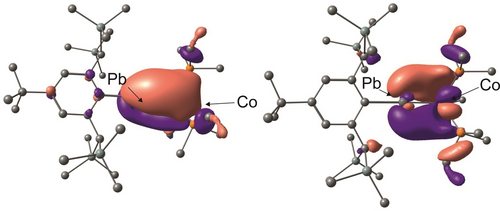
HOMO and HOMO-1 of complex 2 (contour value 0.03).
In the 119Sn NMR spectrum of 1 a, 1 b a signal at 1100, 1027 ppm and in the case of 2 a 207Pb NMR signal at 5022 ppm were observed (Table 2). Both signals lie in the range of known tetrylidyne complexes: Mo≡E (Sn 1021 ppm, Pb 9660 ppm), Rh≡E (Sn 1149 ppm, Pb 5729 ppm).3l, 8 In both cases 1 a (i-CTbb-Sn 179.7 ppm) and 2 (i-CTbb-Pb 248.1 ppm) the signal at highest frequency in the 13C NMR spectrum belongs to the carbon atom attached to the Group 14 element. This high frequency shift in the 13C NMR of tetrylidyne complexes was already documented in the literature for a variety of examples. In the case of plumbylidyne complexes signals at even larger frequencies were observed (280.6, 279.1, 278.7 ppm).3d, 4c, 8 These high frequency signals can be rationalized by the influence of the lead atom on the neighboring atoms.17
|
1 a* |
1 b* |
2* |
5 |
6 |
|---|---|---|---|---|---|
δ 119Sn/207Pb |
1100 |
1027 |
5022 |
61 |
178 |
δ 31P |
14.6 |
14.9 |
35.6 |
9.2 |
-4.4 |
- *119Sn and 207Pb NMR signals are very broad, which is probably due to the anisotropy of the chemical shift caused by the linear coordination geometry at tin or lead atoms, although contributions from the directly bound 59Co-atom cannot be ruled out.
The water addition to the Co≡Sn triple bond was carried out in reaction with five equivalents of the water adduct BaCl2⋅H2O (Scheme 2). With smaller amounts of BaCl2⋅H2O we do not observe a complete product formation. The addition product of two equivalents of water [TbbSn(OH)2CoH2(PMe3)3] (5) was isolated in high yield (87 %) and pale-yellow crystals suitable for X-ray diffraction were obtained from n-pentane at rt (Figure 3). Tobita and co-workers have studied the addition of alcohols to a W≡Ge triple bond found in [Cp*(CO)2WGe{C(SiMe3)3}] and observed formation of a Ge−OR and W−H bond.11b The water molecules react two times with the [Co≡Sn]-moiety by splitting two O−H bonds. Remarkably, a cobalt dihydride and a tin dihydroxide are formed. The Co−Sn bond is elongated and comparable with literature examples for single bonds [2.471(1), 2.437(3) Å] (Table 3).18 The signal in the 119Sn NMR spectrum at 61 ppm is indicative for an increase of the coordination number at tin in comparison to 1 a. The signal of the dihydride moiety was observed at −15.87 ppm and exhibits tin satellites with a coupling constant of 39 Hz. The Sn(OH)2 moiety, which does not tend to oligomerize under water condensation can be compared with the tin trihydroxide found by Kubiak and co-workers as a capping unit of a trinuclear nickel-cluster.19
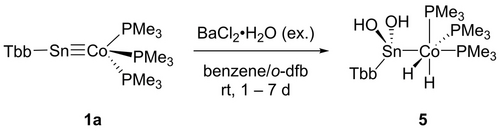
Water addition to 1 a (o-dfb=o-difluorobenzene).
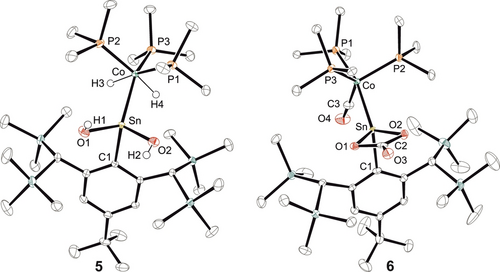
ORTEP of the molecular structures of 5 and 6. Thermal ellipsoids are shown at 50 % probability level. Hydrogen atoms except Co−H and O−H have been omitted.
|
5 |
6 |
|---|---|---|
Sn−Co |
2.4462(4) |
2.4629(8) |
C1−Sn |
2.192(2) |
2.172(5) |
C1−Sn−Co |
125.7(1) |
128.0(1) |
Co−P1 |
2.1908(7) |
2.193(2) |
Co−P2 |
2.1753(7) |
2.227(2) |
Co−P3 |
2.1600(8) |
2.233(2) |
Co−H1 |
1.50(5) |
|
Co−H2 |
1.39(4) |
|
Sn−O1 |
2.032(2) |
2.106(4) |
Sn−O2 |
2.028(2) |
2.108(4) |
Co−C3 |
|
1.728(6) |
C2−O1 |
|
1.320(7) |
C2−O2 |
|
1.335(7) |
C2−O3 |
|
1.235(7) |
C3−O4 |
|
1.168(7) |
Treating a benzene solution of the cobalt stannylidyne 1 a at rt with an excess of carbon dioxide a carbonyl coordination compound [TbbSn(CO3)Co(CO)(PMe3)3] (6) was isolated as the product of a redox reaction in a yield of 62 % (Scheme 3). Obviously, two CO2 molecules react with one cobalt-tin complex under formation of one equivalent of carbon monoxide, as the product of reduction, together with a carbonate. The tin atom is oxidized and exhibits [CO3]2− coordination with Sn−O bond lengths of 2.106(4), 2.108(4) Å which are similar to [{2,6-(Me2NCH2)2C6H3}(Ph)SnCO3] and [Ni3(μ-PPh2CH2PPh2)3(μ3-Cl)(μ3-Sn(OH)(η2-O2CO))] [Sn−O: 2.099(3)–2.121(3) Å].19, 20 The addition and reduction of CO2 in reaction with low valent main group compounds is known from a variety of examples: amidodigermyne,21 carbene stabilized disilicon,22 disilyne bisphosphine adduct,23 disilene,24 dialumene bis(NHC) adduct25 and a base stabilized diborene.26 The [2+2]-cycloaddition of CO2 in reaction with a platinum germylene complex yields a Ge−O and Pt−C bond.27 We therefore discuss as the first step in a possible mechanistic sequence the formation of a [2+2]-cycloaddition product, which results in the complete splitting of a C−O unit and formation of the carbonyl-coordination at cobalt. The intermediately formed Sn=O unit immediately reacts with a further equivalent of CO2 under formation of the carbonate ligand (see SI). The found Co−Sn bond length of 2.4629(8) Å is in line with a single bond between these elements and can be compared with the distance observed in 5. The signal in the 119Sn NMR spectrum at 178 ppm indicates an increase of the coordination number in comparison to 1 a. In the IR spectrum the CO unit exhibits a signal at 1919 cm−1 which can be compared with carbonyl complexes [MeCo(PMe3)3CO] 1881 cm−1; [HCo(PPh3)3CO] 1910 cm−1.28
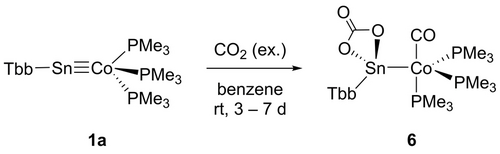
CO2 addition to 1 a.
The protonation of complex 1 a was probed in reaction with [H(OEt2)2][BArF4] (Scheme 4). The molecular structure of the protonated tin complex [TbbSn=CoH(PMe3)3][BArF4] (7 a) is shown in Figure 4 and exhibits a structure comparable with the Ir−Sn structure [TbbSn=IrH(PMe3)3][BArF4] reported recently.29 The Co−Sn bond [2.2907(7) Å] is only slightly elongated in comparison to the triple bond found in 1 a.12, 14 Upon protonation, the angle C1−Sn=Co [156.8(1)°, 152.1(1)°] of the cation of 7 a is reduced and comparable to the C−E=Ir angle found in the protonated iridium complexes [E = Ge 158.8(4)°, E = Sn 159.2(1)°].29 The trigonal bipyramidal coordination sphere of the cobalt atom in the cation of 7 a is also comparable with the ligand arrangement of the homologous iridium-cations [TbbE=IrH(PMe3)3][BArF4] (E = Ge, Sn).29 Protonation of the lead complex 2 yields a protonated species exhibiting a quartet in the 1H NMR spectrum at −21.06 ppm showing 207Pb satellites. We interpret this signal as an indicator for a hydrido-cobaltoplumbylene with a protonation at the cobalt atom [TbbPbCoH(PMe3)3][BArF4] (8). X-ray quality crystals of 8 have not been obtained but NMR evidence suggests formation of a similar structure to 7 and 9. Synthesis of protonated germanium 9 and tin 7 b complexes was also achieved by oxidation of 3 and 4 in reaction with [Ph3C][BArF4] (Scheme 4).
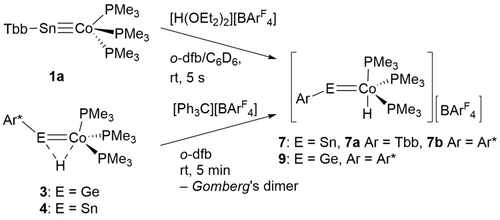
Protonation of 1 a and oxidation of 3 and 4.
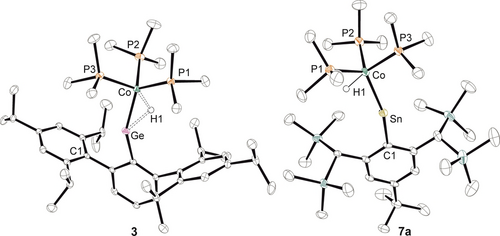
ORTEP of the molecular structures of 3 and the cation of 7 a. Thermal ellipsoids are shown at 50 % probability level. Hydrogen atoms except Co−H have been omitted. Metal hydride positions were found in the difference-Fourier map and refined except for compound 7 a where the M−H distance was restrained to 1.45 Å.
The Co−Ge bond length of 2.1918(4) Å found in 3 (Figure 4, Table 4) is a short bond between these atoms. Literature examples exhibit short Co−Ge bond lengths in a range of 2.1967(6)–2.310(7) Å.30 Cell parameters of compound 4 are almost identical to those of 3, but due to poor crystal quality of 4, structural data of 4 cannot be discussed.
|
3 (E=Ge) |
7 a (E=Sn) |
|---|---|---|
E−Co |
2.1918(4) |
2.2907(7) |
C1−E |
2.005(2) |
2.126(4) |
E−H1 |
1.72(3) |
2.15(5) |
C1−E−Co |
145.2(1) |
156.8(1) |
Co−P1 |
2.1872(8) |
2.200(1) |
Co−P2 |
2.1696(8) |
2.182(1) |
Co−P3 |
2.1931(8) |
2.183(1) |
Co−H1 |
1.56(3) |
1.46(5) |
In the 31P NMR spectrum of 7 a, only one signal was observed, indicating fluxional behavior of 7 a in solution. This phenomenon is similar to the homologous iridium cations.29 The signals in the 119Sn NMR spectrum at 1202 ppm and 1H NMR spectrum at −14.76 ppm (q+satellites, Co−H, 2JSn−H=327 Hz, 2J31P−H=15.7 Hz) of 7 a are in line with the molecular structure. The bonding in 7 a was evaluated by DFT calculations (wB97X-def2-SVP/TZVP(Co,Sn)). The Co−Sn σ-bond is based on the Sn-lone pair σ-donation into a sd-hybrid orbital at the cobalt atom and the π-backdonation stems from a cobalt d-orbital into an empty tin p-orbital. The HOMO of this cation resembles the Sn−Co π-bond and the LUMO shows to a high extent an empty p-orbital at tin (see Figures SI56-57). The NPA charges (Sn: +1.24, Co: −0.46) corroborate the assignment of a cationic tin atom and are in line with the [TbbSnIrH(PMe3)3]-cation (NPA=natural population analysis).29 Thus, the electronic situation of 7 a can be compared with the homologous [IrH=Sn]-cation.29 However, the chemistry of the [CoH=Sn]-cation has not been investigated so far.
In conclusion, organodibromo stannate and plumbate [TbbEBr2]− (E=Sn, Pb) react with the anionic phosphine complex [Co(PMe3)4]− by substitution of a phosphine ligand against a TbbE-cation thus forming the unprecedented Co≡E triple bonds. Cobalt stannylidyne is also accessible via hydrogen atom abstraction from paramagnetic arylhydridostannylene cobalt complex [Ar*SnH=Co(PMe3)3]. The cobalt stannylidyne shows a double addition of water and a redox reaction with carbon dioxide. Furthermore, the cobalt atom of the stannylidyne complex is protonated in reaction with a strong Brønsted acid and the tin-cobalt vinyl cation [TbbSn=CoH(PMe3)3]+ was isolated. Syntheses of analogous tin and germanium cations has also been carried out by oxidizing the organohydrido tetrylene cobalt complexes [Ar*EH=Co(PMe3)3] (E=Ge, Sn). Thus homologous [CoH=E] vinyl cations of the highly reactive [IrH=E] cations have been made accessible and are available for reactivity studies.
Acknowledgments
M. A. thanks the Fonds der Chemischen Industrie for a scholarship. We acknowledge support of the state of Baden-Württemberg through bwHPC and the German research Foundation (DFG) through grant no. INST 40/575-1 FUGG (Justus 2 cluster). Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.