Ex situ Generation of Thiazyl Trifluoride (NSF3) as a Gaseous SuFEx Hub**
Bing-Yu Li
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Search for more papers by this authorKexin Su
Biomolecular Architecture, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, PO Box 2404, 3001 Leuven (Heverlee), Belgium
Search for more papers by this authorProf. Dr. Luc Van Meervelt
Biomolecular Architecture, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, PO Box 2404, 3001 Leuven (Heverlee), Belgium
Search for more papers by this authorProf. Dr. Steven H. L. Verhelst
Laboratory of Chemical Biology, Department of Cellular and Molecular Medicine, KU Leuven, O&N I bis, Herestraat 49, box 901, 3000 Leuven, Belgium
Leibniz Institute for Analytical Sciences ISAS, e.V., Otto-Hahn-Str. 6b, 44227 Dortmund, Germany
Search for more papers by this authorDr. Ermal Ismalaj
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
CIC biomaGUNE, Basque Research and Technology Alliance (BRTA) Paseo Miramon, 20014 San Sebastian, Guipuzcoa, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Wim M. De Borggraeve
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Dr. Joachim Demaerel
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Laboratory of Chemical Biology, Department of Cellular and Molecular Medicine, KU Leuven, O&N I bis, Herestraat 49, box 901, 3000 Leuven, Belgium
Search for more papers by this authorBing-Yu Li
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Search for more papers by this authorKexin Su
Biomolecular Architecture, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, PO Box 2404, 3001 Leuven (Heverlee), Belgium
Search for more papers by this authorProf. Dr. Luc Van Meervelt
Biomolecular Architecture, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, PO Box 2404, 3001 Leuven (Heverlee), Belgium
Search for more papers by this authorProf. Dr. Steven H. L. Verhelst
Laboratory of Chemical Biology, Department of Cellular and Molecular Medicine, KU Leuven, O&N I bis, Herestraat 49, box 901, 3000 Leuven, Belgium
Leibniz Institute for Analytical Sciences ISAS, e.V., Otto-Hahn-Str. 6b, 44227 Dortmund, Germany
Search for more papers by this authorDr. Ermal Ismalaj
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
CIC biomaGUNE, Basque Research and Technology Alliance (BRTA) Paseo Miramon, 20014 San Sebastian, Guipuzcoa, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Wim M. De Borggraeve
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Dr. Joachim Demaerel
Sustainable Chemistry for Metals and Molecules (SCM2), Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Box 2404, 3001 Leuven, Belgium
Laboratory of Chemical Biology, Department of Cellular and Molecular Medicine, KU Leuven, O&N I bis, Herestraat 49, box 901, 3000 Leuven, Belgium
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2023-l4ztm).
Graphical Abstract
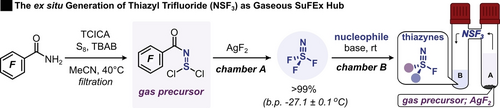
Here we report a synthetic procedure for the efficient ex situ generation of thiazyl trifluoride gas (N≡SF3) as a new Sulfur(VI)-Fluoride Exchange hub. In typical SuFEx fashion, this triple-bonded azasulfur(VI) fluoride reagent and its mono-substituted derivatives react highly effectively with various nucleophiles to deliver a library of unreported thiazynes.
Abstract
Sulfur(VI)-fluoride exchange (SuFEx) chemistry, an all-encompassing term for substitution events that replace fluoride at an electrophilic sulfur(VI), enables the rapid and flexible assembly of linkages around a SVI core. Although a myriad of nucleophiles and applications works very well with the SuFEx concept, the electrophile design has remained largely SO2-based. Here, we introduce S≡N-based fluorosulfur(VI) reagents to the realm of SuFEx chemistry. Thiazyl trifluoride (NSF3) gas is shown to serve as an excellent parent compound and SuFEx hub to efficiently synthesize mono- and disubstituted fluorothiazynes in an ex situ generation workflow. Gaseous NSF3 was evolved from commercial reagents in a nearly quantitative fashion at ambient conditions. Moreover, the mono-substituted thiazynes could be extended further as SuFEx handles and be engaged in the synthesis of unsymmetrically disubstituted thiazynes. These results provide valuable insights into the versatility of these understudied sulfur functionalities paving the way for future applications.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305093-sup-0001-misc_information.pdf21.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dong, J. E. Moses, Chem. Soc. Rev. 2019, 48, 4731–4758.
- 2J. Dong, L. Krasnova, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2014, 53, 9430–9448.
- 3
- 3aS. Li, G. Li, B. Gao, S. P. Pujari, X. Chen, H. Kim, F. Zhou, L. M. Klivansky, Y. Liu, H. Driss, D. D. Liang, J. Lu, P. Wu, H. Zuilhof, J. Moses, K. B. Sharpless, Nat. Chem. 2021, 13, 858–867;
- 3bY. Chao, A. Krishna, M. Subramaniam, D. D. Liang, S. P. Pujari, A. C. Sue, G. Li, F. M. Miloserdov, H. Zuilhof, Angew. Chem. Int. Ed. 2022, 61, e202207456;
- 3cD.-D. Liang, D. E. Streefkerk, D. Jordaan, J. Wagemakers, J. Baggerman, H. Zuilhof, Angew. Chem. Int. Ed. 2020, 59, 7494–7500;
- 3dD. Gahtory, R. Sen, S. Pujari, S. Li, Q. Zheng, J. E. Moses, K. B. Sharpless, H. Zuilhof, Chem. Eur. J. 2018, 24, 10550–10556;
- 3eC. Veryser, J. Demaerel, V. Bieliunas, P. Gilles, W. M. De Borggraeve, Org. Lett. 2017, 19, 5244–5247.
- 4
- 4aL. H. Jones, J. W. Kelly, RSC Med. Chem. 2020, 11, 10–17;
- 4bP. Martín-Gago, C. A. Olsen, Angew. Chem. Int. Ed. 2019, 58, 957–966;
- 4cT. S.-B. Lou, M. C. Willis, Nat. Chem. Rev. 2022, 6, 146–162;
- 4dL. H. Jones, ACS Med. Chem. Lett. 2018, 9, 584–586;
- 4eC. Lee, A. J. Cook, J. E. Elisabeth, N. C. Friede, G. M. Sammis, N. D. Ball, ACS Catal. 2021, 11, 6578–6589;
- 4fS. Wilson Lucas, R. Zijian Qin, K. P. Rakesh, K. S. Sharath Kumar, H. L. Qin, Bioorg. Chem. 2023, 130, 106227;
- 4gM. C. Giel, C. J. Smedley, J. E. Moses, Click Chemistry, Vol. 2021/4, 1st ed., Georg Thieme, Stuttgart, 2022.
- 5
- 5aG. J. Brighty, R. C. Botham, S. Li, L. Nelson, D. E. Mortenson, G. Li, C. Morisseau, H. Wang, B. D. Hammock, K. B. Sharpless, J. W. Kelly, Nat. Chem. 2020, 12, 906–913;
- 5bF. Liu, H. Wang, S. Li, G. A. L. Bare, X. Chen, C. Wang, J. E. Moses, P. Wu, K. B. Sharpless, Angew. Chem. Int. Ed. 2019, 58, 8029–8033;
- 5cS. Kitamura, Q. Zheng, J. L. Woehl, A. Solania, E. Chen, N. Dillon, M. V. Hull, M. Kotaniguchi, J. R. Cappiello, S. Kitamura, V. Nizet, K. B. Sharpless, D. W. Wolan, J. Am. Chem. Soc. 2020, 142, 10899–10904;
- 5dS. Greed, E. L. Briggs, F. I. M. Idiris, A. J. P. White, U. Lucking, J. A. Bull, Chem. Eur. J. 2020, 26, 12533–12538;
- 5eH. Mukherjee, J. Debreczeni, J. Breed, S. Tentarelli, B. Aquila, J. E. Dowling, A. Whitty, N. P. Grimster, Org. Biomol. Chem. 2017, 15, 9685–9695;
- 5fD. D. Liang, D. E. Streefkerk, D. Jordaan, J. Wagemakers, J. Baggerman, H. Zuilhof, Angew. Chem. Int. Ed. 2020, 59, 7494–7500;
- 5gZ.-X. Zhang, M. C. Willis, Chem 2022, 8, 1137–1146;
- 5hS. Greed, O. Symes, J. A. Bull, Chem. Commun. 2022, 58, 5387–5390.
- 6S. Li, P. Wu, J. E. Moses, K. B. Sharpless, Angew. Chem. Int. Ed. 2017, 56, 2903–2908.
- 7Structures containing a formal S≡N triple bond are called “thiazyl” compounds, e.g. thiazyl trifluoride (NSF3, Ref. [8a]). The alternative term “thiazyne” is first coined by Clifford et al. (Ref. [8b]). Our suggestion is that the term “thiazyl” be used in reference to the S≡N fragment, in keeping with the nomenclature of “sulfonyl” or “sulfuryl” species to refer to −SO2−. The term “thiazyne” is used to refer to the compounds as a whole, preferentially to C−S bond-containing derivatives, in line with the term “sulfone”. Yet another term found in the literature for the S≡N triple bond is “sulfanenitrile”, although this is not adopted here.
- 8
- 8aO. Glemser, R. Mews, Angew. Chem. Int. Ed. Engl. 1980, 19, 883–899;
- 8bA. F. Clifford, J. L. Howell, D. L. Wooton, J. Fluorine Chem. 1978, 11, 433–439.
- 9Some non-synthetic applications of NSF3 and derivatives have come from the field of coordination chemistry: U. Behrens, E. Lork, J. Petersen, A. Waterfeld, R. Mews, Z. Anorg. Allg. Chem. 1997, 623, 1518–1524.
- 10O. Glemser, H. Schröder, Z. Anorg. Allg. Chem. 1956, 284, 97–100.
- 11O. Glemser, H. Richert, Z. Anorg. Allg. Chem. 1961, 307, 313–327.
- 12W. H. Kirchhoff, E. B. Wilson, J. Am. Chem. Soc. 1962, 84, 334–336.
- 13D. R. Lide, D. E. Mann, R. M. Fristrom, J. Chem. Phys. 1957, 26, 734–739.
- 14T. Yoshimura, E. Takata, T. Miyake, C. Shimasaki, K. Hasegawa, E. Tsukurimichi, Chem. Lett. 1992, 21, 2213–2216.
- 15X. Yu, H. Hou, B. Wang, J. Phys. Chem. 2018, 122, 3462–3469.
- 16No information on toxicity of NSF3 could be found in the literature or in databases.
- 17Notable works by Yoshimura on the reactivity of diaryl fluorothyazines:
- 17aY. Toshiaki, K. Hiroshi, T. Kyu, T. Eiichi, H. Kiyoshi, S. Choichiro, T. Eiichi, Chem. Lett. 1992, 21, 1433–1436;
- 17bT. Fujii, S. Asai, T. Okada, W. Hao, H. Morita, T. Yoshimura, Tetrahedron Lett. 2003, 44, 6203–6205.
- 18Other recent work has proposed or even observed S≡N triple bonded intermediates, but did not study any fluorinated derivatives:
- 18aO. Glemser, W. Koch, Z. Naturforsch. B 1968, 23, 745–745;
- 18bJ.-F. Lohier, T. Glachet, H. Marzag, A.-C. Gaumont, V. Reboul, Chem. Commun. 2017, 53, 2064–2067;
- 18cE. L. Briggs, A. Tota, M. Colella, L. Degennaro, R. Luisi, J. A. Bull, Angew. Chem. Int. Ed. 2019, 58, 14303–14310;
- 18dW. Hao, T. Fujii, T. Dong, Y. Wakai, T. Yoshimura, Heteroat. Chem. 2004, 15, 193–198;
- 18eP. Wu, J. Demaerel, D. Kong, D. Ma, C. Bolm, Org. Lett. 2022, 24, 6988–6992.
- 19J. Deng, M. Peng, Z. Gao, Y. Wang, B. Wang, W. Zhou, R. Peng, Y. Luo, RSC Adv. 2020, 10, 2740–2746.
- 20
- 20aA. F. Clifford, C. S. Kobayashi, Inorg. Chem. 1965, 4, 571–574;
- 20bA. F. Clifford, J. W. Thompson, Inorg. Chem. 1966, 5, 1424–1427.
- 21O. Glemser, H. Meyer, A. Haa, Chem. Ber. 1964, 97, 1704–1709.
- 22B.-Y. Li, L. Voets, R. Van Lommel, F. Hoppenbrouwers, M. Alonso, S. H. L. Verhelst, W. M. De Borggraeve, J. Demaerel, Chem. Sci. 2022, 13, 2270–2279.
- 23J. Demaerel, C. Veryser, W. M. De Borggraeve, React. Chem. Eng. 2020, 5, 615–631.
- 24
- 24aS. D. Friis, A. T. Lindhardt, T. Skrydstrup, Acc. Chem. Res. 2016, 49, 594–605;
- 24bP. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp, T. Skrydstrup, J. Am. Chem. Soc. 2011, 133, 6061–6071.
- 25G. S. Borovikova, E. S. Levchenko, E. I. Borovik, Zh. Org. Khim. 1979, 15, 2485–2490.
- 26While we assume this to be a two-electron oxidative process, we have little data to support any mechanistic hypotheses. Very recent work proposes a radical mechanism for the AgF2-mediated fluorination of sulfur functional groups: T. Gatzenmeier, Y. Liu, M. Akamatsu, T. Okazoe, K. Nozaki, ChemRxiv 2023, https://doi.org/10.26434/chemrxiv-2023-jzn11.
10.26434/chemrxiv-2023-jzn11 Google Scholar
- 27G. Giacomelli, L. De Luca, G. Nieddu, Synlett 2005, 223–226.
- 28I. V. Koval’, Russ. J. Org. Chem. 2001, 37, 297–317.
- 29H. Richert, O. Glemser, Z. Anorg. Allg. Chem. 1961, 307, 328–344.
- 30H. Tervonen, J. Saunavaara, L. P. Ingman, J. Jokisaari, J. Phys. Chem. B 2006, 110, 16232–16238.
- 31T. Guo, G. Meng, X. Zhan, Q. Yang, T. Ma, L. Xu, K. B. Sharpless, J. Dong, Angew. Chem. Int. Ed. 2018, 57, 2605–2610.
- 32Deposition Numbers 2240276 (for 19), and 2240277 (for 26) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 33S. Li, P. Wu, J. E. Moses, K. B. Sharpless, Angew. Chem. Int. Ed. 2017, 56, 2903–2908.