Ligand-Enabled NiII-Catalyzed Hydroxylarylation of Alkenes with Molecular Oxygen
Dao-Ming Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchuan Lu, Shanghai, 200062 P. R. China
Search for more papers by this authorLi-Qin She
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorHao Yuan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorDr. Yichen Wu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorProf. Dr. Yong Tang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchuan Lu, Shanghai, 200062 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, 310024 P. R. China
CAS Key Laboratory of Energy Regulation Materials, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorDao-Ming Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchuan Lu, Shanghai, 200062 P. R. China
Search for more papers by this authorLi-Qin She
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorHao Yuan
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorDr. Yichen Wu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorProf. Dr. Yong Tang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchuan Lu, Shanghai, 200062 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, 310024 P. R. China
CAS Key Laboratory of Energy Regulation Materials, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai, 200032 P. R. China
Search for more papers by this authorGraphical Abstract
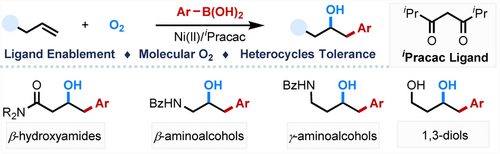
Ni-catalyzed hydroxylarylation of alkenes has been realized with highly efficient and excellent regioselectivity employing molecular oxygen as the oxidant and hydroxyl source. This reaction features mild conditions, broad substrate scope and incredible heterocycle compatibility, providing a variety of β-hydroxylamides, γ-hydroxylamides, β-aminoalcohols, γ-aminoalcohols, and 1,3-diols in high yields.
Abstract
The use of molecular oxygen as the terminal oxidant in transition metal catalyzed oxidative process is an appealing and challenging task in organic synthetic chemistry. Here, we report a Ni-catalyzed hydroxylarylation of unactivated alkenes enabled by a β-diketone ligand with high efficiency and excellent regioselectivity employing molecular oxygen as the oxidant and hydroxyl source. This reaction features mild conditions, broad substrate scope and incredible heterocycle compatibility, providing a variety of β-hydroxylamides, γ-hydroxylamides, β-aminoalcohols, γ-aminoalcohols, and 1,3-diols in high yields. The synthetic value of this methodology was demonstrated by the efficient synthesis of two bioactive compounds, (±)-3′-methoxyl citreochlorol and tea catechin metabolites M4.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202304573-sup-0001-misc_information.pdf21.3 MB | Supporting Information |
| anie202304573-sup-0001-mj20057_(ccdc_2252793).cif977.6 KB | Supporting Information |
| anie202304573-sup-0001-mj23162_(ccdc_2252795).cif877.9 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH.-J. Arpe, Industrial Organic Chemistry, 5th ed. (translated by H.-J. Arpe, S. Hawkins), Wiley-VCH, Weinheim, 2010;
- 1bM. B. Smith, J. March, March's Advanced Organic Chemistry, 5th ed, Wiley, New York, 2001;
- 1cComprehensive Organic Functional Group Transformations II, Vol. 2, (Eds.: A. R. Katrinsky, R. J. K. Taylor), Elsevier, Amsterdam, 2005.
- 2For selected reviews on hydration of alkenes using Mukaiyama-type protocols
- 2aT. Mukaiyama, T. Yamada, Bull. Chem. Soc. Jpn. 1995, 68, 17;
- 2bS. W. M. Crossley, C. Obradors, R. M. Martinez, R. A. Shenvi, Chem. Rev. 2016, 116, 8912;
- 2cR. W. Hoffmann, Chem. Soc. Rev. 2016, 45, 577;
- 2dS. L. Shevick, C. V. Wilson, S. Kotesova, D. Kim, P. L. Holland, R. A. Shenvi, Chem. Sci. 2020, 11, 12401.
- 3For selected examples on Mukaiyama-type hydration reaction, see:
- 3aA. Zombeck, D. E. Hamilton, R. S. Dargo, J. Am. Chem. Soc. 1982, 104, 6782;
- 3bT. Mukaiyama, S. Isayama, S. Inoki, K. Kato, T. Yamada, T. Takai, Chem. Lett. 1989, 18, 449;
- 3cS. Isayama, T. Mukaiyama, Chem. Lett. 1989, 18, 1071;
- 3dS. Inoki, K. Kato, S. Isayama, T. Mukaiyama, Chem. Lett. 1990, 19, 1869;
- 3eT. Hashimoto, D. Hirose, T. Taniguchi, Angew. Chem. Int. Ed. 2014, 53, 2730;
- 3fC. Obradors, R. M. Martinez, R. A. Shenvi, J. Am. Chem. Soc. 2016, 138, 4962.
- 4For selected examples on the application of Mukaiyama-type hydration reaction, see:
- 4aR. A. Shenvi, C. A. Guerrero, J. Shi, C.-C. Li, P. S. Baran, J. Am. Chem. Soc. 2008, 130, 7241;
- 4bH. Ishikawa, D. A. Colby, S. Seto, P. Va, A. Tam, H. Kakei, T. J. Rayl, I. Hwang, D. L. Boger, J. Am. Chem. Soc. 2009, 131, 4904;
- 4cO. F. Jeker, E. M. Carreira, Angew. Chem. Int. Ed. 2012, 51, 3474;
- 4dH. Renata, Q. Zhou, P. S. Baran, Science 2013, 339, 59;
- 4eX. Hu, T. J. Maimone, J. Am. Chem. Soc. 2014, 136, 5287;
- 4fD. N. Tran, N. Cramer, Chem. Eur. J. 2014, 20, 10654;
- 4gD. Zhu, B. Yu, J. Am. Chem. Soc. 2015, 137, 15098;
- 4hS. Kawamura, H. Chu, J. Felding, P. S. Baran, Nature 2016, 532, 90;
- 4iP. Yang, M. Yao, J. Li, Y. Li, A. Li, Angew. Chem. Int. Ed. 2016, 55, 6964;
- 4jM. A. Baker, R. M. Demoret, M. Ohtawa, R. A. Shenvi, Nature 2019, 575, 643.
- 5For selected examples on carbon radical-initiated oxycarbo-functionalization of alkenes with O2 as the radical oxidant:
- 5aA. Dickschat, A. Studer, Org. Lett. 2010, 12, 3972;
- 5bT. Taniguchi, Y. Sugiura, H. Zaimoku, H. Ishibashi, Angew. Chem. Int. Ed. 2010, 49, 10154;
- 5cY. Su, X. Sun, G. Wu, N. Jiao, Angew. Chem. Int. Ed. 2013, 52, 9808;
- 5dS. Kindt, H. Jasch, M. R. Heinrich, Chem. Eur. J. 2014, 20, 6251;
- 5eY.-H. Chen, M. Lee, Y.-Z. Lin, D. Leow, Chem. Asian J. 2015, 10, 1618;
- 5fY. Yang, Y. Liu, Y. Jiang, Y. Zhang, D. A. Vicic, J. Org. Chem. 2015, 80, 6639;
- 5gL. Li, M. Huang, C. Liu, J.-C. Xiao, Q.-Y. Chen, Y. Guo, Z.-G. Zhao, Org. Lett. 2015, 17, 4714;
- 5hR. K. M. Khan, Y. Zhao, T. D. Scully, S. L. Buchwald, Chem. Eur. J. 2018, 24, 15215;
- 5iQ. Li, W. Fan, D. Peng, B. Meng, S. Wang, R. Huang, S. Liu, S. Li, ACS Catal. 2020, 10, 4012;
- 5jS. R. Chowdhury, D. Singh, I. U. Hoque, S. Maity, J. Org. Chem. 2020, 85, 13939;
- 5kB. Sun, R. Zhu, X. Zhuang, X. Shi, P. Huang, Z. Yan, C. Yu, C. Jin, Org. Lett. 2021, 23, 617.
- 6For selected examples on carbon radical-initiated Meerwein-type oxycarbofunctionalization:
- 6aM. R. Heinrich, A. Wetzel, M. Kirschstein, Org. Lett. 2007, 9, 3833;
- 6bM. Hartmann, Y. Li, A. Studer, J. Am. Chem. Soc. 2012, 134, 16516;
- 6cG. Fumagalli, S. Boyd, M. F. Greaney, Org. Lett. 2013, 15, 4398;
- 6dC.-J. Yao, Q. Sun, N. Rastogi, B. König, ACS Catal. 2015, 5, 2935;
- 6eS. Kindt, K. Wicht, M. R. Heinrich, Angew. Chem. Int. Ed. 2016, 55, 8744;
- 6fE. Yamaguchi, W. Tanaka, A. Itoh, Chem. Asian J. 2019, 14, 121;
- 6gJ. Wang, L. Xue, M. Hong, B. Ni, T. Niu, Green Chem. 2020, 22, 411;
- 6hL.-M. Altmann, V. Zantop, P. Wenisch, N. Diesendorf, M. R. Heinrich, Chem. Eur. J. 2021, 27, 2452.
- 7For selected examples on transition metal-catalyzed intramolecular oxyarylation of alkenes:
- 7aJ. P. Wolfe, M. A. Rossi, J. Am. Chem. Soc. 2004, 126, 1620;
- 7bM. B. Hay, J. P. Wolfe, J. Am. Chem. Soc. 2005, 127, 16468;
- 7cG. Zhang, L. Cui, Y. Wang, L. Zhang, J. Am. Chem. Soc. 2010, 132, 1474;
- 7dC. Zhu, J. R. Falck, Angew. Chem. Int. Ed. 2011, 50, 6626;
- 7eR. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2012, 51, 1926;
- 7fB. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505;
- 7gB. A. Hopkins, Z. J. Garlets, J. P. Wolfe, Angew. Chem. Int. Ed. 2015, 54, 13390;
- 7hG. M. Borrajo-Calleja, V. Bizet, C. Mazet, J. Am. Chem. Soc. 2016, 138, 4014;
- 7iN. Hu, K. Li, Z. Wang, W. Tang, Angew. Chem. Int. Ed. 2016, 55, 5044.
- 8For selected examples on transition metal-catalyzed intermolecular oxyarylation of alkenes:
- 8aA. D. Melhado, W. E. Brenzovich Jr., A. D. Lackner, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 8885;
- 8bL. T. Ball, M. Green, G. C. Lloyd-Jones, C. A. Russell, Org. Lett. 2010, 12, 4724;
- 8cS. Kirchberg, R. Fröhlich, A. Studer, Angew. Chem. Int. Ed. 2010, 49, 6877;
- 8dA. D. Satterfield, A. Kubota, M. S. Sanford, Org. Lett. 2011, 13, 1076;
- 8eL. T. Ball, G. C. Lloyd-Jones, C. A. Russell, Chem. Eur. J. 2012, 18, 2931;
- 8fV. Ramella, Z. He, C. G. Daniliuc, A. Studer, Org. Lett. 2015, 17, 664;
- 8gM. J. Harper, E. J. Emmett, J. F. Bower, C. A. Russell, J. Am. Chem. Soc. 2017, 139, 12386;
- 8hS. Zhang, C. Wang, X. Ye, X. Shi, Angew. Chem. Int. Ed. 2020, 59, 20470.
- 9Y. Wang, C. Lin, Z. Zhang, L. Shen, B. Zou, Org. Lett. 2023, 25, 2172. Also see: D.-M. Wang, L.-Q. She, H. Yuan, Y. Wu, P. Wang, ChemRxiv preprint 2023, https://doi.org/10.26434/chemrxiv-2023-0kcb5.
10.26434/chemrxiv-2023-0kcb5 Google Scholar
- 10
- 10aD.-M. Wang, W. Feng, Y. Wu, T. Liu, P. Wang, Angew. Chem. Int. Ed. 2020, 59, 20399;
- 10bD.-M. Wang, L.-Q. She, Y. Wu, C. Zhu, P. Wang, Nat. Commun. 2022, 13, 6878;
- 10cJ.-P. Wang, S. Song, Y. Wu, P. Wang, Nat. Commun. 2022, 13, 5059.
- 11K. Wang, Z. Ding, Z. Zhou, W. Kong, J. Am. Chem. Soc. 2018, 140, 12364.
- 12Deposition numbers 2252795 (for 3 a), 2252793 (for 8 v) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 13
- 13aJ. Derosa, O. Apolinar, T. Kang, V. T. Tran, K. M. Engle, Chem. Sci. 2020, 11, 4287;
- 13bX. Qi, T. Diao, ACS Catal. 2020, 10, 8542;
- 13cY.-C. Luo, C. Xu, X. Zhang, Chin. J. Chem. 2020, 38, 1371;
- 13dH.-Y. Tu S Zhu, F. L. Qing L Chu, Synthesis 2020, 52, 1346;
- 13eL. M. Wickham, R. Giri, Acc. Chem. Res. 2021, 54, 3415.
- 14
- 14aR. Sunnapu, G. Rajendar, Eur. J. Org. Chem. 2021, 1637;
- 14bR. Sunnapu, S. N. Banoth, R. S. Reyno, A. Thomas, N. Venugopal, G. Rajendar, J. Org. Chem. 2020, 85, 4103;
- 14cR. J. Bold, S. Virudachalam, D. J. McConkey, J. Surg. Res. 2001, 100, 11;
- 14dJ. D. Lambert, J. E. Rice, J. Hong, Z. Houa, C. S. Yang, Bioorg. Med. Chem. Lett. 2005, 15, 873.
- 15
- 15aT. Yamada, K. Takahashi, K. Kato, T. Takai, S. Inoki, T. Mukaiyama, Chem. Lett. 1991, 20, 641;
- 15bT. Mukaiyama, T. Takai, T. Yamada, O. Rhode, Chem. Lett. 1990, 19, 1661.
- 16
- 16aO. Gutierrez, J. C. Tellis, D. N. Primer, G. A. Molander, M. C. Kozlowski, J. Am. Chem. Soc. 2015, 137, 4896;
- 16bV. M. Chernyshev, V. P. Ananikov, ACS Catal. 2022, 12, 1180.