Chemically Gradient Hydrogen-Bonded Organic Framework Crystal Film
Graphical Abstract
We introduce a novel chemical gradient strategy to fabricate a crystal-deposited HOF on an in situ grown polymer film. The fabricated film showed versatility in chemical bonding along its thickness from covalent to hydrogen-bonded network and showed improved proton conductivity compared to its polymeric analog.
Abstract
Hydrogen-bonded organic frameworks (HOFs) are ordered supramolecular solid structures, however, nothing much explored as centimetre-scale self-standing films. The fabrication of such crystals comprising self-supported films is challenging due to the limited flexibility and interaction of the crystals, and therefore studies on two-dimensional macrostructures of HOFs are limited to external supports. Herein, we introduce a novel chemical gradient strategy to fabricate a crystal-deposited HOF film on an in situ-formed covalent organic polymer film (Tam-Bdca-CGHOF). The fabricated film showed versatility in chemical bonding along its thickness from covalent to hydrogen-bonded network. The kinetic-controlled Tam-Bdca-CGHOF showed enhanced proton conductivity (8.3×10−5 S cm−1) compared to its rapid kinetic analogue, Tam-Bdca-COP (2.1×10−5 S cm−1), which signifies the advantage of bonding-engineering in the same system.
Introduction
The current century has witnessed rapid multi-directional developments of various open framework materials such as covalent organic frameworks (COFs) and hydrogen-bonded organic frameworks (HOFs).1, 2 The nature of the chemical linkage among building blocks differentiates these classes of materials from each other. Notably, HOFs are supramolecular solids built by intermolecular hydrogen bonding.3 In particular, the flexibility of supramolecular networks in HOFs makes them suitable as smart porous materials with immense potential applications like chiral separations,4 proton conduction,5 sensing, etc.6 The weak, but reversible hydrogen-bonding interactions in HOFs make them more ordered and provide for large extended networks compared to covalently bonded solids. Notably, the precise arrangement of frameworks is a critical factor in many applications to improve material performance. Despite these merits, studies on the much-desired macrostructure feasibility of HOFs have been confined mostly to single crystals and support polymer-assisted mixed matrix membranes.7 To the best of our knowledge, little research has been done on free-standing macroscopic forms of HOF.8 The poor coherence between the long-range and ordered single crystals of HOFs is the major hurdle impeding the fabrication of its higher-order physical forms. Considering these strenuous challenges in the fabrication of centimetre-scale practical films of HOFs, we put forward a novel strategy to grow HOF crystals on its intrinsic chemical composite support. The technique proposes in situ construction of a covalent organic polymer (COP) supported HOF film through kinetically controlled interfacial synthesis: both COP and HOF were built from the same organic linkers, i.e diatopic aldehyde (Bdca; 4,4′-biphenyldicarboxaldehyde) and tetratopic amine (Tam; tetrakis-4-aminophenylmethane) using the Schiff base imine condensation reaction. Herein, the control of reaction kinetics allowed for uniform growth of HOF microcrystals on the surface of the in situ formed COP film at the liquid-liquid interface, which yielded a chemical gradient self-standing thin film (Tam-Bdca-CGHOF) of a few centimetres (2–3 cm). The tuning of chemical bonding in the macrostructures of open frameworks shall provide exciting functional features.9 The chemical gradience alters the functional properties at the micro-level of the macroscopic forms which can be applied for mass and charge transfer applications. Remarkably, the Tam-Bdca-CGHOF offered an improvement (≈4 times) ) in proton conduction (8.3×10−5 S cm−1) compared to a rapid-kinetic analogue of the same building blocks (Tam-Bdca-COP) obtained at the interface (2.1×10−5 S cm−1).
Results and Discussion
The free-standing and chemically gradient HOF film (Tam-Bdca-CGHOF) was fabricated through p-toulenesulphonic acid (PTSA) catalyzed interfacial polymerization reaction (Figure 1a,b, and c; S-2). Specifically, Tam-Bdca-CGHOF was synthesized at the interface of ethyl acetate: water solvents. The aqueous solution (10 ml) of Tam (0.039 mmol) and the PTSA catalyst (0.15 mmol; 4 equivalent to Tam) was allowed to interface with the organic solution (10 ml) of Bdca (0.078 mmol) at room temperature for 12 days. The reaction was carried out in a closed system (beaker with a cover) for the first 6 days (Figure 1a), which lead to the formation of a transparent film. Furthermore, the reaction was allowed to proceed in a partially open condition (a tiny hole was made in the lid) for the next 6 days under kinetic control to form HOFs. Finally, the thin film obtained at the interface was collected and washed with water, N, N-dimethylacetamide (DMA), acetone, and chloroform. The centimetre-scale pale yellow colour Tam-Bdca-CGHOFshowed a self-standing ability after washing and drying (Figure 1f and Figure S1). Importantly, when we turned to a rapid-kinetic interface synthesis using the same building block by reducing the equivalency of PTSA (2 equivalent to Tam) and reaction time (3 days) we end up obtaining pure COP film (Tam-Bdca-COP) (S-2, Supporting Information).
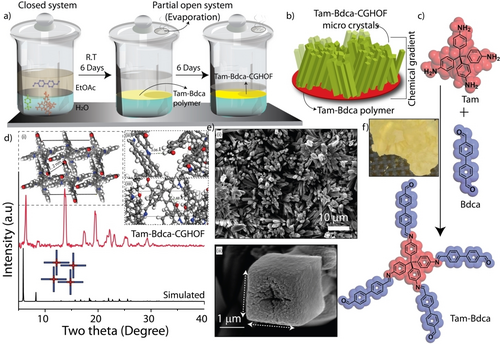
a) The graphical representation of the kinetically controlled interfacial synthesis of Tam-Bdca-CGHOF. b) The graphical representation of HOF crystal deposited film of Tam-Bdca-CGHOF. c) The Scheme of the Schiff base reaction of Tam and Bdca into Tam-Bdca oligomers. Herein the chemical structure Tam-Bdca represents the oligomer of HOF. d) The experimental PXRD profile of Tam-Bdca-CGHOF with simulated PXRD and (i) corresponding 3D model shows (ii) C−H−N and (iii) C−H−O bonding. (e) (i) The SEM image of Tam-Bdca-CGHOF film and (ii) SEM image of quasi-square shaped crystal f) The digital photograph of Tam-Bdca-CGHOF.
The structural periodicity of Tam-Bdca-CGHOF was investigated with the help of powder X-ray diffraction (PXRD) analysis. The PXRD profile of Tam-Bdca-CGHOF showed sharp crystalline peaks at two theta 6.3°, 8.7°, 13.7°, 17.4°, 19.4°, 22.1°, 23.0°, 25.2°, and 29.0° (Figure 1d). The presence of these sharp peaks indicated the long-range order networks in the film. Notably, the obtained PXRD was similar to that of previously reported XRD of a single crystal of Tam-Bdca, thereby validating the origin of peaks10 (from the HOF crystals in the film). Although COF is an expected product from the symmetric organic linkers, the theoretical models suggested the HOF model as the best fit compared to the various COF models (Figure S2–S4). In COF models, the organic framework is connected through covalent bonds (C=N) which yielded a crystalline 3D polymer. Whereas in the HOF model, the oligomers are linked through intermolecular hydrogen-bonding and form a supramolecule (Figure 1c–d). Furthermore, the absence of any monomer peaks in the PXRD profile signified the purity of the thin film (Figure S5). The slight variations in the experimental and simulated PXRD (Tam-Bdca-HOF) could be due to the overall chemical composition of the Tam-Bdca-CGHOF which contains both COP and HOF. Moreover, HOF crystals may be present as polycrystalline particles as well as micrometer-sized single crystals in the film. Meanwhile, the rapid-kinetic analogue (Tam-Bdca-COP) yielded an amorphous PXRD profile signifying no formation of HOF crystals (Figure S6). The driving force for the formation of the HOF network originated from the unreacted aldehyde (−CHO) and imine (C=N) functional pockets in the Tam-Bdca oligomers (FigureS7). The lone pair electrons of the carbonyl oxygen (C=O) hydrogen-bonded with aromatic hydrogen of the adjacent oligomer in the same thread (d[C−H⋅⋅⋅O]=2.48 Å). In addition, the lone pair of imine nitrogen can interact with the hydrogen of the carbonyl group of adjacent thread (d[C−H⋅⋅⋅N]=3.063 Å). Both C−H⋅⋅⋅O and C−H⋅⋅⋅N interactions created a self-assembly of Tam-Bdca oligomers into 3D-network with quasi-tetragonal-shaped pores. The unique crystal packing also results in one-dimensional quasi-tetragonal microcrystals (Figure 1e).
The disappearance of monomers is evident in the Fourier-transform infrared (FT-IR) spectra of Tam-Bdca-CGHOF (Figure S8). The absence of amine (N−H) vibration peaks at 3150 cm−1 implied complete tetratopic imine condensation of the Tam moieties. The presence of an imine (C=N) bond is evident from the observed stretching vibration at 1619 cm−1 (Figure 2c). The unreacted end of Bdca linker shows the corresponding aldehyde (C=O) stretching peak at 1693 cm−1. Meanwhile, the rapid-kinetic Tam-Bdca-COP showed a strong C=N stretching peak (1621 cm−1) compared to the slow-kinetic Tam-Bdca-CGHOF (Figure S8). Moreover, the unreacted C=O in Tam-Bdca-COP appeared at 1697 cm−1 with less intensity. The slightly lower stretching frequency of C=O in Tam-Bdca-CGHOF compared to Tam-Bdca-COP could be due to the participation of oxygen in the intermolecular hydrogen bonding. The observation emphasizes the critical role of slow reaction kinetics to infuse a hydrogen-bonded network into the film. In our work, rapid-kinetic imine condensation reaction always led to a complete covalent-bonded polymer.
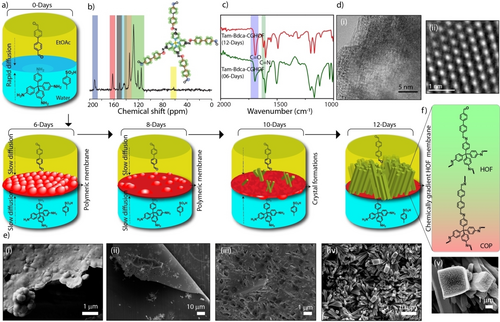
a) The graphical representation of the formation mechanism of Tam-Bdca-CGHOF at the interface of water and ethyl acetate. It illustrates the COP and subsequent HOF formation during the progress of reaction on 6th, 8th, 10th, and 12th days. b) Solid-state 13C-CP MAS NMR of Tam-Bdca-CGHOF. c) FT-IR profiles of Tam-Bdca-CGHOF show the difference in intensity of C=O and C=N bonds during the progress of the reaction. d) TEM image of Tam-Bdca-CGHOF at different scales: (i) 5 nm and (ii) 1 nm. e) The SEM images of Tam-Bdca-CGHOF during the progress of the reaction: (i) 6th day (ii) 8th day (iii) 10th day (iv) 12th day and (v) zoomed view of crystals. f) Molecular representation of chemical gradience in Tam-Bdca-CGHOF.
Furthermore, the atomic level details of Tam-Bdca-CGHOF were decoded from solid-state nuclear magnetic resonance (NMR) spectroscopy.11 The 13C cross-polarization magic-angle-spinning (CP MAS) NMR spectra indicate the presence of aldehyde carbon (*C=O) and imine carbon(*C=N) at 191 and 162 ppm, respectively (Figure 2b). Moreover, the carbon atoms from benzene cores (from Tam and Bdca) are distributed from the chemical shift 154 to 108 ppm. Similarly, the solid-state 1H NMR provided further details of the protons in the oligomer (Figure S9). The aldehyde and imine protons resonated at 9.9 and 8.2 ppm, respectively. Both 1D 13C and 1H NMR suggested the formation of the Tam-Bdca oligomer. Moreover, to confirm the peak assignments of Tam-Bdca-CGHOF, we have performed advanced 1H/13C frequency shifted Lee–Goldburg HETCOR (1H/13C FSLG HETCOR) NMR.12 The 2D NMR profile provided the spatial proximities of 13C and 1H of Tam-Bdca-CGHOF. The polarization features of carbons and protons in the HETCOR NMR profile clearly validate the peak shift assignment of aldehyde, imine, and aromatic atoms in mono-dimensional 1H and 13C CP/MAS NMR profiles (Figure S9).
The matrix-assisted laser desorption/ionization fourier-transform ion cyclotron resonance mass spectrometry (MALDI-FT-ICR-MS) of Tam-Bdca film was analyzed to confirm the presence of expected HOF oligomeric fractions. Notably, the MALDI-TOF-MS showed a peak at 1149.5 m/z, which matches the molecular mass of the HOF oligomer in Tam-Bdca-CGHOF (Figure S10–S11).To understand the general aspect of the oligomer formation, we have reacted terephthaldehyde (Ta) C2 linker (instead of bdca) with Tam at the liquid-liquid interface for the construction of a film (Tam-Ta) (Figure S12). The basic characterizations (PXRD and FT-IR) of Tam-Ta are given in the Supporting Information (Figure S12). Notably, the MALDI-FT-ICR-MS indicated the presence of oligomeric Tam-Ta (845 m/z) in the resulting film (Figure S13). However, Tam-Ta film was severely broken compared to Tam-Bdca-CGHOF.
The thermogravimetric analysis (TGA) showed excellent thermal stability of Tam-Bdca-CGHOF up to 400°C with ≈11 % loss of mass (Figure S14). The porosity of Tam-Bdca-CGHOF was analyzed through the Ar gas adsorption at 77 K (Figure S15). A low Brunauer–Emmett–Teller (BET) surface area (≈15 m2 g−1) was observed from the adsorption isotherm, whereas the CO2 adsorption analysis of Tam-Bdca-CGHOF showed an uptake capacity of up to 513 μmol g−1 at 298 K and 1 bar (Figure S16). The lower uptake of Ar could be due to the narrow aperture of Tam-Bdca-CGHOF.13 The non-local density functional theory (NLDFT) pore size distribution showed a pore size of≈1.2 nm, in line with the theoretically modelled HOF structure (Figure S17). The transmission electron microscopic images (TEM) showed the nano-level assembly of Tam-Bdca-CGHOF (Figure 2d and Figure S18). Predominantly, a 2D layer-like morphology was observed with the presence of several lattice fringes indicating the periodic arrangement of oligomers in Tam-Bdca-CGHOF. The bright spots on selected area electron diffraction (SAED) further revealed the ordered network of Tam-Bdca-CGHOF. Importantly, the scanning electron microscopic (SEM) images further revealed microscopic assembling details of Tam-Bdca-CGHOF. It shows slightly inclined perpendicularly oriented single crystals on the surface of the COP thin film (Figure 1e, 2e, and Figure S19). The single crystals (micro crystals) are cuboidal in shape with a height of ≈10–20 μm and lateral sides of ≈4–5 μm. These microcrystals are uniformly distributed over the entire in situ thin film support. Overall, the Tam-Bdca-CGHOF is composed of polymer thin film support and microcrystals of HOF oligomers (Figure S20).
Interestingly, the entire hybrid film is made up of the same monomers (Tam and Bdca), however, the nature of bonding (covalent and hydrogen bonding) between the monomers and molecular level arrangements (either to form amorphous COP in the beginning and later formation of self-assembled HOF) induced a chemical gradience across Tam-Bdca-CGHOF. The covalently bonded thin film base could be readily differentiated from the hydrogen-bonded network of elongated microcrystals in SEM images. Moreover, the stability of integrated microcrystals in the chemical gradient Tam-Bdca-CGHOF in solvents including water and highly polar DMA (used for washing the film) implies robust interactions between the HOF and COP. These interactions could be due to the possible weak chemical bonding such as hydrogen bonding and aromatic pi-pi interactions between the polymers and HOF oligomers in the system. The similar chemical constituents HOF and COP create coherence between these two phases and the chemical gradience (polymeric to hydrogen-bonded network) stabilizes the HOF crystals on the COP surface like a root-supported plant.
Several interactions are possible in the chemical gradient film including covalent bonding, hydrogen bonding, and pi-pi interactions. To understand the bonding nature and decipher the deeper mechanism of formation of the Tam-Bdca-CGHOF, we characterized the growth of the film at different time intervals by using FT-IR, PXRD, and SEM techniques (Figure 2a). The interface reaction was monitored on the 6th, 8th, 10th, and 12th days. No physically transferrable film was observed at the interface until the 5th day of reaction. The formation of a very thin transparent film was noticed on the 6th day. It was collected on a copper foil surface and subjected to further characterization. The FT-IR revealed the chemical bonding nature of the film (Figure 2c and Figure S21). Both C=O and C=N intensities were similar on the 6th day, which indicates the extended imine network in the film. Interestingly, the intensity of the C=O stretching peak was increased from the 6th to the 12th day signifying the increasing number of unreacted aldehydes. Notably, the 6th-day film showed the stretching frequency at 1696 cm−1 which shifted to a lower frequency of 1693 cm−1 on the 12th day. This slightly lower wave number shifting could be due to the participation of oxygen of C=O in hydrogen-bonding interactions as an electron donor. In PXRD analysis, the initially formed 6th and 8th-day films showed amorphous nature whereas the film obtained on the 10th day displayed crystallinity with a slight difference in peak positions compared to the 12th-day film (Figure S22). It indicates the formation of the ordered arrangement of oligomers on the surface of the pre-formed amorphous film during the second half of the reaction period. The PXRD profiles at different reaction times suggested an initial formation of the amorphous film followed by slow HOF crystals formation on the COP surface. Furthermore, the morphological evolution of Tam-Bdca-CGHOF was verified through SEM at different time intervals which provided better visibility and understanding of the chemical gradience (Figure 2e and Figure S23). The initially formed thin film was composed of oval-shaped spheres until the film thickness grow with time to become a planar surface. The crystalline characteristics were observed on the pre-formed COP film on the 8th day while full deposition of crystals was achieved on the 12th day. The continuous low kinetics of the interfacial reaction (initial fully closed system to later slow evaporation partial open system) facilitated the formation of a chemical gradience across the Tam-Bdca film (Figure 2f). The possible cause for chemical gradience could be attributed to the following suggested mechanism (Figure S24): 1) Initially, the aldehyde and amine-PTSA solutions (solvent-solvent biphase) were in direct contact and forming a polymeric network (COP), which created an additional phase (solid film) in the system; 2) As a result, the formed solid mid-phase acted as an in situ separator of monomers within two solutions; 3) The COP solid-phase grew in thickness with the progress of the reaction and restricted the concentration of amine passage into the aldehyde solution; 4) The lower number of amines and increasing concentration of aldehydes (through slow evaporation of ethylacetate) produced a favourable environment for the formation of HOF oligomers. The slow evaporation also helps for the uniform distribution of microcrystals on the film. Whereas, rapid evaporation (interfacial synthesis at the complete open condition) results in fewer and more scattered distribution of crystals on the film. The FT-IR suggested the oligomeric formations with the presence of C=N and C=O bonds (Figure S25a) and PXRD indicates the crystalline nature of the deposited oligomers (Figure S25b). However, poor integrity between crystals and film was noticed during the solvent (DMA) washing. Furthermore, the SEM images clearly indicate the scattered distribution of these crystals on the film (Figure S25c). Moreover, the catalyst concentration controlled kinetic study was performed to understand the critical role of slow-reaction kinetics to produce the chemical gradience. The PTSA and −NH2 will react and forms a salt with a lower nucleophilicity compared to a free amine.14 The lower equivalency of PTSA (1 or 2 equivalence to Tam) results in the rapid formation of the amorphous polymeric film due to the availability of free −NH2 groups (Figures S6 and S8).
HOFs are known for their proton conductivity due to the presence of the hydrogen bonding network (Figure 3a).5 The proton conductivity of Tam-Bdca-CGHOF was investigated to analyze the effect of induction of hydrogen bonding in the film. The Tam-Bdca-CGHOF film was taken directly for measuring the proton conductivity from the Nyquist plot under electric potential (Figure 3b and Figure S26–S28). The proton conductivity analysis was carried out from 313 K to 363 K at 95 % of humidity. The conductivity increased with temperature from 313 K to 353 K and then decreased at 363 K (Figure 3c). The highest proton conductivity (8.3×10−5 S cm−1) of Tam-Bdca-CGHOF was observed at 353 K, while the Tam-Bdca-COP showed a much lower proton conductivity (2.1×10−5 S cm−1) at 353 K. This illustrated the ability of self-integrated HOFs to improve the proton conductivity in Tam-Bdca-CGHOF. The recyclability measurement of Tam-Bdca-CGHOF showed ≈100 % recovery of proton conductivity after drying the sample. Each measurement was analyzed for three consecutive impedance cycles (Figure S29). The poor proton conductivity of Tam-Bdca-CGHOF in anhydrous condition shows the unavailability of inherent protons for the transfer. Again, Tam-Bdca-CGHOF showed a lower activation energy of 0.14 eV compared to Nafion (0.22 eV) (Table S1–S2).15 It is important to note that the Tam-Bdca-CGHOF used for proton conductivity measurement was free of any doping and external supports.
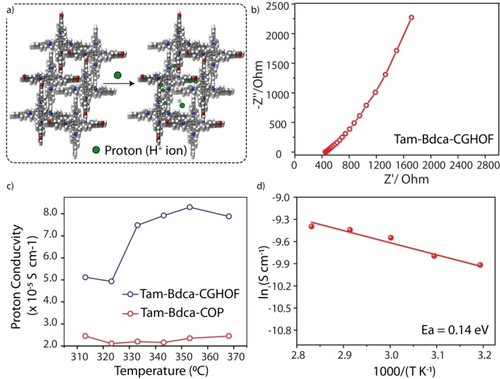
a) Model representation of proton conductivity inTam-Bdca. b) The impedance profile of chemically gradient Tam-Bdca-CGHOF. c) The comparison plot of the proton conductivity Vs temperature of Tam-Bdca-CGHOF and Tam-Bdca-COP. d) Arrhenius plot of chemically gradient Tam-bdca-CGHOF.
Conclusion
In conclusion, the study demonstrates an effective strategy to fabricate a chemically gradient HOF over COP film (Tam-Bdca-CGHOF) by controlling the reaction kinetics. In this method, the in situ synthesized polymeric membrane reduced the monomer diffusion through the interface and created an environment for the growth of HOF crystals. The Tam-Bdca-CGHOF showed chemical gradience across the film thickness. The self-integrated crystals on the film were seen to be stable and showed strong adherence to the film. The improved proton conductivity of Tam-Bdca-CGHOF compared to its polymeric analog revealed its hydrogen bonding effect. The chemical features of the films can be employed in potential applications, which will be part of our further investigations.
Acknowledgments
AKM and DS acknowledge Khalifa University Abu Dhabi for the generous support of this research. This research is supported by ASPIRE, the technology program management pillar of Abu Dhabi's Advanced Technology Research Council (ATRC), via the ASPIRE AARE 436 (AARE20-123). M.A.A. thanks the Materials Chemistry Consortium for computational resources on YOUNG (EP/P020194).
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.





