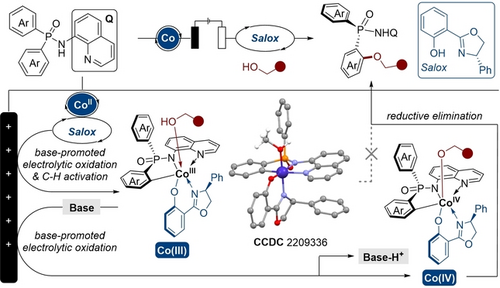
Base-Promoted Electrochemical CoII-catalyzed Enantioselective C−H Oxygenation
Gang Zhou
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorJia-Hao Chen
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorQi-Jun Yao
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorFan-Rui Huang
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorZhen-Kai Wang
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Bing-feng Shi
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorGang Zhou
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorJia-Hao Chen
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorQi-Jun Yao
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorFan-Rui Huang
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorZhen-Kai Wang
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Bing-feng Shi
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorGraphical Abstract
The first electrochemical CoII-catalyzed enantioselective C−H alkoxylation is reported. A broad range of alkoxylated phosphinamides were obtained in good yields with excellent enantioselectivities (up to 98 % yield and >99 % ee). A cobalt(III) alcohol complex was prepared and characterized, and was found to be a key intermediate in this reaction. Mechanistic studies revealed that the oxidation of CoIII to CoIV was facilitated by a base.
Abstract
Metalla-electrocatalyzed C−H oxygenation represents one of the most straightforward and sustainable approaches to access valuable oxygenated molecules. Despite the significant advances, the development of enantioselective electrochemical C−H oxygenation reaction is very challenging and remains elusive. Herein, we described the first electrochemical CoII-catalyzed enantioselective C−H alkoxylation. A broad range of enantioenriched alkoxylated phosphinamides were obtained in good yields with excellent enantioselectivities (up to 98 % yield and >99 % ee). An unusual cobalt(III) alcohol complex was prepared and fully characterized, which was proven to be a key intermediate of this C−H alkoxylation reaction. Mechanistic studies revealed that the oxidation of CoIII to CoIV was facilitated by a base and the whole process proceeded through a cobalt(III/IV/II) catalytic cycle.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202302964-sup-0001-3ai.cif787.7 KB | Supporting Information |
| anie202302964-sup-0001-3ai_checkcif.pdf85.8 KB | Supporting Information |
| anie202302964-sup-0001-3al.cif851.2 KB | Supporting Information |
| anie202302964-sup-0001-3al_cifreport.pdf124.8 KB | Supporting Information |
| anie202302964-sup-0001-misc_information.pdf11 MB | Supporting Information |
| anie202302964-sup-0001-[CoIII][MeOH].cif1.2 MB | Supporting Information |
| anie202302964-sup-0001-[CoIII][MeOH]_checkcif.pdf94.6 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Select selected reviews:
- 1aO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 1bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 1cP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879;
- 1dG. Rouquet, N. Chatani, Angew. Chem. Int. Ed. 2013, 52, 11726;
- 1eJ. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754;
- 1fC. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603;
- 1gB. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle, B.-F. Shi, Chem. Rev. 2021, 121, 14957.
- 2For selected examples, see:
- 2aA. R. Dick, K. L. Hull, M. S. Sanford, J. Am. Chem. Soc. 2004, 126, 2300;
- 2bR. Giri, J. Liang, J. G. Lei, J. J. Li, D. H. Wang, X. Chen, I. C. Naggar, C. Guo, B. M. Foxman, J.-Q. Yu, Angew. Chem. Int. Ed. 2005, 44, 7420;
- 2cJ. Roane, O. Daugulis, Org. Lett. 2013, 15, 5842;
- 2dF.-J. Chen, S. Zhao, F. Hu, K. Chen, Q. Zhang, S.-Q. Zhang, B.-F. Shi, Chem. Sci. 2013, 4, 4187;
- 2eR. K. Rit, M. R. Yadav, A. K. Sahoo, Org. Lett. 2014, 16, 968;
- 2fL.-B. Zhang, X.-Q. Hao, S.-K. Zhang, Z.-J. Liu, X.-X. Zheng, J.-F. Gong, J.-L. Niu, M.-P. Song, Angew. Chem. Int. Ed. 2015, 54, 272;
- 2gT. Okada, K. Nobushige, T. Satoh, M. Miura, Org. Lett. 2016, 18, 1150.
- 3For selected reviews on organometallic electrochemical C−H activation, see:
- 3aC. Xu, A. Lei, T.-S. Mei, H.-C. Xu, K. Xu, C.-C. Zeng, CCS Chem. 2022, 4, 1120;
- 3bK. J. Jiao, Y. K. Xing, Q. L. Yang, H. Qiu, T. S. Mei, Acc. Chem. Res. 2020, 53, 300;
- 3cL. Ackermann, Acc. Chem. Res. 2020, 53, 84;
- 3dF. Kakiuchi, T. Kochi, Chem. Lett. 2020, 49, 1256;
- 3eH. Wang, X. Gao, Z. Lv, T. Abdelilah, A. Lei, Chem. Rev. 2019, 119, 6769;
- 3fV. Dwivedi, D. Kalsi, B. Sundararaju, ChemCatChem 2019, 11, 5160;
- 3gM. Yan, Y. Kawamata, P. S. Baran, Chem. Rev. 2017, 117, 13230.
- 4
- 4aY. Q. Li, Q. L. Yang, P. Fang, T. S. Mei, D. Zhang, Org. Lett. 2017, 19, 2905;
- 4bQ. L. Yang, Y. Q. Li, C. Ma, P. Fang, X. J. Zhang, T. S. Mei, J. Am. Chem. Soc. 2017, 139, 3293.
- 5
- 5aN. Sauermann, T. H. Meyer, C. Tian, L. Ackermann, J. Am. Chem. Soc. 2017, 139, 18452;
- 5bT. H. Meyer, J. C. A. Oliveira, D. Ghorai, L. Ackermann, Angew. Chem. Int. Ed. 2020, 59, 10955;
- 5cL. Massignan, X. Tan, T. H. Meyer, R. Kuniyil, A. M. Messinis, L. Ackermann, Angew. Chem. Int. Ed. 2020, 59, 3184;
- 5dX. Tan, L. Massignan, X. Hou, J. Frey, J. C. A. Oliveira, M. N. Hussain, L. Ackermann, Angew. Chem. Int. Ed. 2021, 60, 13264.
- 6A. Shrestha, M. Lee, A. L. Dunn, M. S. Sanford, Org. Lett. 2018, 20, 204.
- 7Y. K. Au, H. Lyu, Y. Quan, Z. Xie, J. Am. Chem. Soc. 2020, 142, 6940.
- 8S. Jin, J. Kim, D. Kim, J.-W. Park, S. Chang, ACS Catal. 2021, 11, 6590.
- 9
- 9aJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 9bC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 9cT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, eaao4798;
- 9dQ. Zhang, B.-F. Shi, Acc. Chem. Res. 2021, 54, 2750;
- 9eC.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2021, 143, 14025;
- 9fT. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 6455;
- 9gO. Vyhivskyi, A. Kudashev, T. Miyakoshi, O. Baudoin, Chem. Eur. J. 2021, 27, 1231;
- 9hQ. Zhang, L.-S. Wu, B.-F. Shi, Chem 2022, 8, 384;
- 9iY. Zheng, C. Zheng, Q. Gu, S.-L. You, Chem Catal. 2022, 2, 2965.
- 10For examples on electrochemical enantioselective C−H activation to form C−C bonds, see:
- 10aU. Dhawa, C. Tian, T. Wdowik, J. C. A. Oliveira, J. Hao, L. Ackermann, Angew. Chem. Int. Ed. 2020, 59, 13451–13457;
- 10bY.-Q. Huang, Z.-J. Wu, L. Zhu, Q. Gu, X. Lu, S.-L. You, T.-S. Mei, CCS Chem. 2022, 4, 3181–3189;
- 10cW. Wei, A. Scheremetjew, L. Ackermann, Chem. Sci. 2022, 13, 2783–2788;
- 10dQ.-J. Yao, F.-R. Huang, J.-H. Chen, M.-Y. Zhong, B.-F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202218533.
- 11
- 11aL. Grigorjeva, O. Daugulis, Angew. Chem. Int. Ed. 2014, 53, 10209;
- 11bO. Daugulis, J. Roane, L. D. Tran, Acc. Chem. Res. 2015, 48, 1053.
- 12J.-H. Chen, M.-Y. Teng, F.-R. Huang, H. Song, Z.-K. Wang, H.-L. Zhuang, Y.-J. Wu, X. Wu, Q.-J. Yao, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202210106.
- 13For examples of CoIII-catalyzed enantioselective C−H activation, see:
- 13aS. Fukagawa, Y. Kato, R. Tanaka, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2019, 58, 1153;
- 13bY.-H. Liu, P.-X. Li, Q.-J. Yao, Z.-Z. Zhang, D.-Y. Huang, M.-D. Le, H. Song, L. Liu, B.-F. Shi, Org. Lett. 2019, 21, 1895;
- 13cK. Ozols, Y. S. Jang, N. Cramer, J. Am. Chem. Soc. 2019, 141, 5675;
- 13dA. Whyte, A. Torelli, B. Mirabi, L. Prieto, J. F. Rodriguez, M. Lautens, J. Am. Chem. Soc. 2020, 142, 9510;
- 13eY.-H. Liu, P.-P. Xie, L. Liu, J. Fan, Z.-Z. Zhang, X. Hong, B.-F. Shi, J. Am. Chem. Soc. 2021, 143, 19112;
- 13fW.-K. Yuan, B.-F. Shi, Angew. Chem. Int. Ed. 2021, 60, 23187;
- 13gK. Ozols, S. Onodera, Ł. Woźniak, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 655;
- 13hY.-B. Zhou, T. Zhou, P.-F. Qian, J.-Y. Li, B.-F. Shi, ACS Catal. 2022, 12, 9806;
- 13iY. Hirata, D. Sekine, Y. Kato, L. Lin, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2022, 61, e202205341.
- 14
- 14aQ.-J. Yao, J.-H. Chen, H. Song, F.-R. Huang, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202202892;
- 14bB.-J. Wang, G.-X. Xu, Z.-W. Huang, X. Wu, X. Hong, Q.-J. Yao, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202208912;
- 14cX.-J. Si, D. Yang, M.-C. Sun, D. Wei, M.-P. Song, J.-L. Niu, Nat. Synth. 2022, 1, 709.
- 15Deposition Numbers 2158235, 2209336 and 2209229 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16
- 16aC. H. Cheon, T. Imahori, H. Yamamoto, Chem. Commun. 2010, 46, 6980;
- 16bY. Miyaki, T. Onishi, H. Kurosawa, Inorg. Chim. Acta 2000, 300–302, 369.
- 17NaOPiv ⋅ H2O was regenerated by the generation of H2 as the sole byproduct through cathodic proton reduction, indicating the sustainable nature of this approach.