Metal-Induced Planar Chirality of Soft-Bridged Binuclear Platinum(II) Complexes: 100 % Phosphorescence Quantum Yields, Chiral Self-Sorting, and Circularly Polarized Luminescence
Corresponding Author
Prof. Jintong Song
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, 610068 China
College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorHui Xiao
Department of Chemistry, Southern University of Science and Technology, Shenzhen, 518000 China
These authors contributed equally to this work.
Search for more papers by this authorBao Zhang
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Methodology (supporting)
Search for more papers by this authorLang Qu
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Methodology (supporting)
Search for more papers by this authorProf. Xiangge Zhou
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Formal analysis (supporting)
Search for more papers by this authorProf. Ping Hu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, 610068 China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Zong-Xiang Xu
Department of Chemistry, Southern University of Science and Technology, Shenzhen, 518000 China
Search for more papers by this authorCorresponding Author
Prof. Haifeng Xiang
College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Jintong Song
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, 610068 China
College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorHui Xiao
Department of Chemistry, Southern University of Science and Technology, Shenzhen, 518000 China
These authors contributed equally to this work.
Search for more papers by this authorBao Zhang
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Methodology (supporting)
Search for more papers by this authorLang Qu
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Methodology (supporting)
Search for more papers by this authorProf. Xiangge Zhou
College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Formal analysis (supporting)
Search for more papers by this authorProf. Ping Hu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, 610068 China
Contribution: Formal analysis (supporting)
Search for more papers by this authorCorresponding Author
Prof. Zong-Xiang Xu
Department of Chemistry, Southern University of Science and Technology, Shenzhen, 518000 China
Search for more papers by this authorCorresponding Author
Prof. Haifeng Xiang
College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorGraphical Abstract
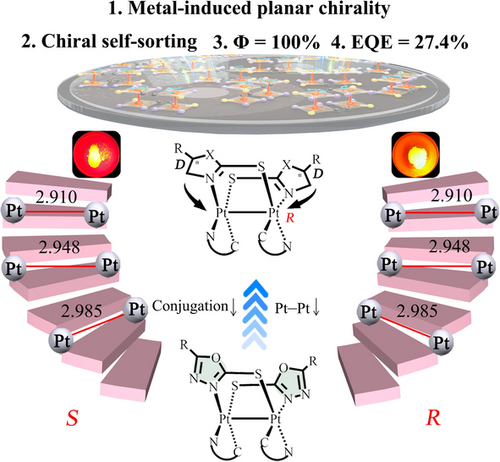
Racemic (R)/(S) and enantiopure (D,R,D)/(L,S,L) binuclear PtII complexes with metal-induced planar chirality were designed and prepared by using soft-bridged achiral and chiral ligands, respectively. Their phosphorescence quantum yields up to 100 % can be achieved by shortening intramolecular Pt−Pt distance for highly efficient solution-processed circularly polarized OLEDs.
Abstract
PtII complexes have attracted a great deal of interest due to their rich phosphorescent properties. However, these square-planar PtII complexes are far more likely to encounter the problems of lack of metal-induced chirality and emission “aggregation-caused quenching”. Herein, soft-bridged binuclear PtII complexes bearing metal-induced planar chirality were synthesized and characterized. These soft bridging ligands with smaller conjugated system would help to not only improve solubility for synthesis and enantioseparation but also introduce point chirality from amino acid for highly efficient diastereoselectivity. Furthermore, the intramolecular Pt−Pt distances could be well regulated by soft bridging ligands, and consequently the phosphorescence quantum yield up to 100 % could be achieved by shortening intramolecular Pt−Pt distance for first time. These complexes can be used as emitters in highly efficient solution-processed organic light-emitting diodes.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202302011-sup-0001-(L,S,L)Pt4a.cif519.8 KB | Supporting Information |
| anie202302011-sup-0001-(Race)Pt1.cif1,006.6 KB | Supporting Information |
| anie202302011-sup-0001-(Race)Pt2.cif531.9 KB | Supporting Information |
| anie202302011-sup-0001-(Race)Pt3a.cif2.3 MB | Supporting Information |
| anie202302011-sup-0001-(Race)Pt3b.cif572.2 KB | Supporting Information |
| anie202302011-sup-0001-misc_information.pdf4.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. W.-W. Yam, V. K.-M. Au, S. Y.-L. Leung, Chem. Rev. 2015, 115, 7589–7728;
- 1bM. A. Baldo, D. F. O′Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson, S. R. Forrest, Nature 1998, 395, 151–154;
- 1cC.-W. Chan, L.-K. Cheng, C.-M. Che, Coord. Chem. Rev. 1994, 132, 87–97;
- 1dF. N. Castellano, I. E. Pomestchenko, E. Shikhova, F. Hua, M. L. Muro, N. Rajapakse, Coord. Chem. Rev. 2006, 250, 1819–1828;
- 1eJ. A. G. Williams, S. Develay, D. L. Rochester, L. Murphy, Coord. Chem. Rev. 2008, 252, 2596–2611;
- 1fE. V. Puttock, M. T. Walden, J. A. G. Williams, Coord. Chem. Rev. 2018, 367, 127–162;
- 1gH. Xiang, J. Cheng, X. Ma, X. Zhou, J. J. Chruma, Chem. Soc. Rev. 2013, 42, 6128–6185;
- 1hL. Murphy, J. A. G. Williams, Top. Organomet. Chem. 2010, 28, 75–111.
- 2
- 2aM. Chaaban, C. Zhou, H. Lin, B. Chyi, B. Ma, J. Mater. Chem. C 2019, 7, 5910–5924;
- 2bS. Horiuchi, K. Umakoshi, Coord. Chem. Rev. 2023, 476, 214924.
- 3J. Kalinowski, V. Fattori, M. Cocchi, J. A. G. Williams, Coord. Chem. Rev. 2011, 255, 2401–2425.
- 4
- 4aM. Lenes, G. Garcia-Belmonte, D. Tordera, A. Pertegás, J. Bisquert, H. J. Bolink, Adv. Funct. Mater. 2011, 21, 1581–1586;
- 4bS. van Reenen, P. Matyba, A. Dzwilewski, R. A. J. Janssen, L. Edman, M. Kemerink, J. Am. Chem. Soc. 2010, 132, 13776–13781;
- 4cQ. Pei, G. Yu, C. Zhang, Y. Yang, A. J. Heeger, Science 1995, 269, 1086–1088;
- 4dW. Lu, M. C. W. Chan, N. Zhu, C.-M. Che, C. Li, Z. Hui, J. Am. Chem. Soc. 2004, 126, 7639–7651;
- 4eB. Pashaei, S. Karimi, H. Shahroosvand, P. Abbasi, M. Pilkington, A. Bartolotta, E. Fresta, J. Fernandez-Cestau, R. D. Costa, F. Bonaccorso, Chem. Soc. Rev. 2019, 48, 5033–5139.
- 5
- 5aJ. Zhao, W. Wu, J. Sun, S. Guo, Chem. Soc. Rev. 2013, 42, 5323–5351;
- 5bJ. Zhao, S. Ji, H. Guo, RSC Adv. 2011, 1, 937–950;
- 5cJ. Zhou, Q. Liu, W. Feng, Y. Sun, F. Li, Chem. Rev. 2015, 115, 395–465.
- 6
- 6aQ. Zhao, F. Li, C. Huang, Chem. Soc. Rev. 2010, 39, 3007–3030;
- 6bY. Feng, J. Cheng, L. Zhou, X. Zhou, H. Xiang, Analyst 2012, 137, 4885–4901;
- 6cY. Yang, Q. Zhao, W. Feng, F. Li, Chem. Rev. 2013, 113, 192–270.
- 7
- 7aV. Fernández-Moreira, F. L. Thorp-Greenwood, M. P. Coogan, Chem. Commun. 2010, 46, 186–202;
- 7bQ. Zhao, C. Huang, F. Li, Chem. Soc. Rev. 2011, 40, 2508–2524.
- 8
- 8aK. Tuong Ly, R.-W. Chen-Cheng, H.-W. Lin, Y.-J. Shiau, S.-H. Liu, P.-T. Chou, C.-S. Tsao, Y.-C. Huang, Y. Chi, Nat. Photonics 2017, 11, 63–68;
- 8bS. Lee, Y. Lee, K. Kim, S. Heo, D. Y. Jeong, S. Kim, J. Cho, C. Kim, Y. You, Inorg. Chem. 2021, 60, 7738–7752;
- 8cD. Saito, T. Ogawa, M. Yoshida, J. Takayama, S. Hiura, A. Murayama, A. Kobayashi, M. Kato, Angew. Chem. Int. Ed. 2020, 59, 18723–18730;
- 8dA. Aliprandi, M. Mauro, L. De Cola, Nat. Chem. 2016, 8, 10–15;
- 8eM. J. Bryant, J. M. Skelton, L. E. Hatcher, C. Stubbs, E. Madrid, A. R. Pallipurath, L. H. Thomas, C. H. Woodall, J. Christensen, S. Fuertes, T. P. Robinson, C. M. Beavers, S. J. Teat, M. R. Warren, F. Pradaux-Caggiano, A. Walsh, F. Marken, D. R. Carbery, S. C. Parker, N. B. McKeown, R. Malpass-Evans, M. Carta, P. R. Raithby, Nat. Commun. 2017, 8, 1800;
- 8fA. K.-W. Chan, M. Ng, Y.-C. Wong, M.-Y. Chan, W.-T. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2017, 139, 10750–10761;
- 8gN. Komiya, M. Okada, K. Fukumoto, D. Jomori, T. Naota, J. Am. Chem. Soc. 2011, 133, 6493–6496;
- 8hM. A. Soto, V. Carta, R. J. Andrews, M. T. Chaudhry, M. J. MacLachlan, Angew. Chem. Int. Ed. 2020, 59, 10348–10352.
- 9
- 9aB. Ma, J. Li, P. I. Djurovich, M. Yousufuddin, R. Bau, M. E. Thompson, J. Am. Chem. Soc. 2005, 127, 28–29;
- 9bB. Ma, P. I. Djurovich, S. Garon, B. Alleyne, M. E. Thompson, Adv. Funct. Mater. 2006, 16, 2438–2446;
- 9cC. Zhou, L. Yuan, Z. Yuan, N. K. Doyle, T. Dilbeck, D. Bahadur, S. Ramakrishnan, A. Dearden, C. Huang, B. Ma, Inorg. Chem. 2016, 55, 8564–8569;
- 9dC. Zhou, Y. Tian, Z. Yuan, M. Han, J. Wang, L. Zhu, M. S. Tameh, C. Huang, B. Ma, Angew. Chem. Int. Ed. 2015, 54, 9591–9595.
- 10
- 10aX. Wu, Y. Liu, Y. Wang, L. Wang, H. Tan, M. Zhu, W. Zhu, Y. Cao, Org. Electron. 2012, 13, 932–937;
- 10bJ. Yu, X. Wu, H. Tan, Y. Liu, Y. Wang, M. Zhu, W. Zhu, Sci. China Chem. 2013, 56, 1137–1142;
- 10cW. Xiong, F. Meng, C. You, P. Wang, J. Yu, X. Wu, Y. Pei, W. Zhu, Y. Wang, S. Su, J. Mater. Chem. C 2019, 7, 630–638;
- 10dM. Chaaban, Y.-C. Chi, M. Worku, C. Zhou, H. Lin, S. Lee, A. Ben-Akacha, X. Lin, C. Huang, B. Ma, Inorg. Chem. 2020, 59, 13109–13116;
- 10eW. Xiong, F. Meng, H. Tan, Y. Wang, P. Wang, Y. Zhang, Q. Tao, S. Su, W. Zhu, J. Mater. Chem. C 2016, 4, 6007–6015;
- 10fX. Wu, D.-G. Chen, D. Liu, S.-H. Liu, S.-W. Shen, C.-I. Wu, G. Xie, J. Zhou, Z.-X. Huang, C.-Y. Huang, S.-J. Su, W. Zhu, P.-T. Chou, J. Am. Chem. Soc. 2020, 142, 7469–7479.
- 11
- 11aA. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau, M. E. Thompson, J. Am. Chem. Soc. 2003, 125, 7377–7387;
- 11bT.-Y. Li, Y.-M. Jing, X. Liu, Y. Zhao, L. Shi, Z. Tang, Y.-X. Zheng, J.-L. Zuo, Sci. Rep. 2015, 5, 14912;
- 11cN. Hellou, M. Srebro-Hooper, L. Favereau, F. Zinna, E. Caytan, L. Toupet, V. Dorcet, M. Jean, N. Vanthuyne, J. A. G. Williams, L. Di Bari, J. Autschbach, J. Crassous, Angew. Chem. Int. Ed. 2017, 56, 8236–8239.
- 12
- 12aE. Anger, M. Rudolph, L. Norel, S. Zrig, C. Shen, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, J. Autschbach, J. Crassous, R. Réau, Chem. Eur. J. 2011, 17, 14178–14198;
- 12bN. Saleh, C. Shen, J. Crassous, Chem. Sci. 2014, 5, 3680–3694;
- 12cX.-P. Zhang, V. Y. Chang, J. Liu, X.-L. Yang, W. Huang, Y. Li, C.-H. Li, G. Muller, X.-Z. You, Inorg. Chem. 2015, 54, 143–152;
- 12dH. Sesolis, J. Dubarle-Offner, C. K. M. Chan, E. Puig, G. Gontard, P. Winter, A. L. Cooksy, V. W. W. Yam, H. Amouri, Chem. Eur. J. 2016, 22, 8032–8037;
- 12eL. Norel, M. Rudolph, N. Vanthuyne, J. A. G. Williams, C. Lescop, C. Roussel, J. Autschbach, J. Crassous, R. Réau, Angew. Chem. Int. Ed. 2010, 49, 99–102;
- 12fJ. R. Brandt, X. Wang, Y. Yang, A. J. Campbell, M. J. Fuchter, J. Am. Chem. Soc. 2016, 138, 9743–9746;
- 12gT. Biet, T. Cauchy, Q. Sun, J. Ding, A. Hauser, P. Oulevey, T. Bürgi, D. Jacquemin, N. Vanthuyne, J. Crassous, N. Avarvari, Chem. Commun. 2017, 53, 9210–9213;
- 12hL. Qu, C. Li, G. Shen, F. Gou, J. Song, M. Wang, X. Xu, X. Zhou, H. Xiang, Mater. Chem. Front. 2019, 3, 1199–1208;
- 12iJ. Song, M. Wang, X. Zhou, H. Xiang, Chem. Eur. J. 2018, 24, 7128–7132;
- 12jJ. Song, M. Wang, X. Xu, L. Qu, X. Zhou, H. Xiang, Dalton Trans. 2019, 48, 4420–4428;
- 12kJ. Hong, S. Kim, G. Park, Y. Lee, H. Kim, S. Kim, T.-W. Lee, C. Kim, Y. You, Chem. Sci. 2021, 12, 8668–8681;
- 12lR. Inoue, R. Kondo, Y. Morisaki, Chem. Commun. 2020, 56, 15438–15441;
- 12mL. Wang, H. Xiao, L. Qu, J. Song, W. Zhou, X. Zhou, H. Xiang, Z.-X. Xu, Inorg. Chem. 2021, 60, 13557–13566;
- 12nC. Shen, E. Anger, M. Srebro, N. Vanthuyne, K. K. Deol, T. D. Jefferson, G. Muller, J. A. G. Williams, L. Toupet, C. Roussel, J. Autschbach, R. Réau, J. Crassous, Chem. Sci. 2014, 5, 1915–1927;
- 12oZ.-P. Yan, X.-F. Luo, W.-Q. Liu, Z.-G. Wu, X. Liang, K. Liao, Y. Wang, Y.-X. Zheng, L. Zhou, J.-L. Zuo, Y. Pan, H. Zhang, Chem. Eur. J. 2019, 25, 5672–5676;
- 12pB. Li, Y. Li, M. H.-Y. Chan, V. W.-W. Yam, J. Am. Chem. Soc. 2021, 143, 21676–21684;
- 12qJ. Morikubo, T. Tsubomura, Inorg. Chem. 2022, 61, 17154–17165.
- 13
- 13aM. Cingi Biagini, M. Ferrari, M. Lanfranchi, L. Marchiò, M. Angela Pellinghelli, J. Chem. Soc. Dalton Trans. 1999, 1575–1580;
- 13bT. R. Schulte, J. J. Holstein, L. Krause, R. Michel, D. Stalke, E. Sakuda, K. Umakoshi, G. Longhi, S. Abbate, G. H. Clever, J. Am. Chem. Soc. 2017, 139, 6863–6866;
- 13cN. Komiya, T. Muraoka, M. Iida, M. Miyanaga, K. Takahashi, T. Naota, J. Am. Chem. Soc. 2011, 133, 16054–16061;
- 13dG. Hirata, Y. Kobayashi, R. Sato, Y. Shigeta, N. Yasuda, H. Maeda, Chem. Eur. J. 2019, 25, 8797–8804;
- 13eB. Yang, G. Zou, S. Zhang, H. Ni, H. Wang, W. Xu, C. Yang, H. Zhang, W. Yu, K. Luo, Angew. Chem. Int. Ed. 2021, 60, 10531–10536;
- 13fR. Inoue, R. Kondo, Y. Morisaki, Chem. Mater. 2022, 34, 7959–7970;
- 13gL. Yuan, Q.-J. Ding, Z.-L. Tu, X.-J. Liao, X.-F. Luo, Z.-P. Yan, Z.-G. Wu, Y.-X. Zheng, Chin. Chem. Lett. 2022, 33, 1459–1462;
- 13hJ. Song, H. Xiao, L. Fang, L. Qu, X. Zhou, Z.-X. Xu, C. Yang, H. Xiang, J. Am. Chem. Soc. 2022, 144, 2233–2244;
- 13iM. Ikeshita, S. Furukawa, T. Ishikawa, K. Matsudaira, Y. Imai, T. Tsuno, ChemistryOpen 2022, 11, e202100277.
- 14
- 14aK.-W. Lo, G. S. M. Tong, G. Cheng, K.-H. Low, C.-M. Che, Angew. Chem. Int. Ed. 2022, 61, e202115515;
- 14bM. Xue, T.-L. Lam, G. Cheng, W. Liu, K.-H. Low, L. Du, S. Xu, F.-F. Hung, D. L. Phillips, C.-M. Che, Adv. Opt. Mater. 2022, 10, 2200741.
- 15Deposition numbers 2221585 (for (Race)Pt1), 2221586 (for (Race)Pt2), 2221587 (for (Race)Pt3b), 2221588 (for (Race)Pt3a) and 2221589 (for (L,S,L)Pt4a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16
- 16aY. Hong, J. W. Y. Lam, B. Z. Tang, Chem. Soc. Rev. 2011, 40, 5361–5388;
- 16bX. Ma, R. Sun, J. Cheng, J. Liu, F. Gou, H. Xiang, X. Zhou, J. Chem. Educ. 2016, 93, 345–350.
- 17
- 17aJ. L. Greenfield, J. Wade, J. R. Brandt, X. Shi, T. J. Penfold, M. J. Fuchter, Chem. Sci. 2021, 12, 8589–8602;
- 17bL. Arrico, L. Di Bari, F. Zinna, Chem. Eur. J. 2021, 27, 2920–2934;
- 17cF. Zinna, G. Pescitelli, L. Di Bari, Chirality 2020, 32, 765–769;
- 17dG. Mazzeo, S. Ghidinelli, R. Ruzziconi, M. Grandi, S. Abbate, G. Longhi, ChemPhotoChem 2022, 6, e202100222.
- 18
- 18aY. Tao, C. Yang, J. Qin, Chem. Soc. Rev. 2011, 40, 2943–2970;
- 18bK. S. Yook, J. Y. Lee, Adv. Mater. 2014, 26, 4218–4233;
- 18cR. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon, M. E. Thompson, Appl. Phys. Lett. 2003, 82, 2422–2424.
- 19A. Turak, RSC Adv. 2013, 3, 6188–6225.