Adaptive Catalytic Systems for Chemical Energy Conversion
Graphical Abstract
While the use of biomass, CO2, and green hydrogen has reached a significant level of maturity, the need to re-consider our ways of operating chemical production processes becomes obvious. The fluctuation associated with renewable energy and feedstock holds challenges for catalysts to cope with the resulting dynamics. However, new opportunities arise once catalyst design starts to aim at performance that is adaptive rather than task-specific.
Abstract
The rapidly growing importance of green hydrogen and renewable carbon resources as essential feedstocks for sustainable chemical value chains opens room for disruptive innovations regarding chemical production processes. The fluctuation and variability associated with non-fossil energy and raw material supply holds many challenges for catalysts to cope with the resulting dynamics. However, many new opportunities also arise once catalyst design starts to aim at performance that is “adaptive” rather than “task-specific”. In this Scientific Perspective, we propose to define adaptivity in catalysis on the basis of three essential properties that are reversibility, rapidity, and robustness (R3 rule). Promising design strategies and selected examples are described to substantiate the scientific concept and to highlight its potential for chemical energy conversion.
1 Motivation
In a climate neutral future energy-chemistry nexus, the de-carbonisation of the electricity sector defines challenges, but also major opportunities for the defossilisation of the chemical value chain.1 Using hydrogen (H2) as molecular pivot for the conversion of non-fossil carbon feedstocks provides the basis for technologies to store, distribute, and harvest renewable energy enabling prosperity for an ever growing global population. The carbon content of CO2, biomass, and recycled plastics can thus be exploited for the production of fuels, base chemicals, fine chemicals, agrochemicals, and pharmaceuticals.2 The dynamic deployment of cost-effective technologies generating electricity from renewable sources and the growing environmental and societal pressure for products with low-carbon intensity are bringing into sight the tipping point to establish novel business cases via carbon circularity. On a fundamental molecular level, the controlled activation of dihydrogen and its transfer to organic substrates define the key elementary processes for the necessary de- and re-functionalization of the non-fossil carbon sources.
Extensive efforts in science and technology are currently dedicated to the development of multifunctional catalytic systems for selective hydrogenation and hydrogenolysis reactions opening such new synthetic strategies.3 While many of these catalysts present outstanding properties, they are typically developed to fulfill one specific task for a given substrate with maximum activity and selectivity under precisely defined parameters following established concepts of chemical production (Figure 1a). For post-fossil value chains, however, the ability to cope with the dynamics of alternative energy resources and with quality variations of chemical feedstocks will become increasingly important. This is already widely discussed for the design of next generation manufacturing plants.4 In particular, so-called modular multi-purpose/multi-product plants are attracting considerable attention to enable agile and efficient hybrid production concepts.4a, 4b Variations in production and electricity supply are addressed through the implementation of modules that can be reconfigured depending on the needs on the macroscopic level.44 As a complementary approach, we intend to highlight in this Scientific Perspective the opportunity to address the challenges of diversity and variability in energy and feedstock supply using a molecular approach through the development of adaptive catalytic systems.

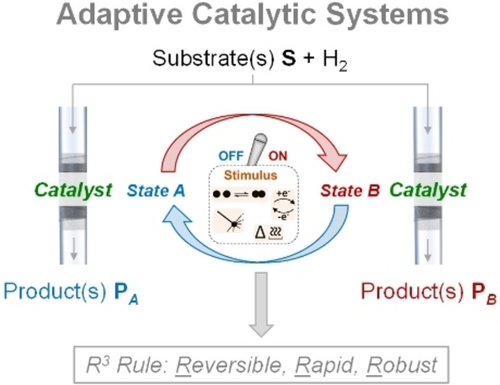
Comparison between static (a), and adaptive (b) catalytic systems exemplified for the selective hydrogenation of a substrate S to two different products PA and PB. While two different specialized catalysts A and B would be applied in the static approach, the adaptive catalyst can be reversibly switched between two states to direct the reaction to products PA or PB. The interconversion of the two states is stimulated by a chemical or physico-chemical process.
The term adaptive is generally defined as “having an ability to change to suit changing conditions”5 and is used in various connotations ranging from biological and material sciences to economics and social sciences, as well as even in colloquial language. For catalysis and catalytic processes, we introduce the term with the following definition:
Adaptive catalytic systems are capable of adjusting or being adjusted into different states of their performance in response to dynamic changes in the reactive environment. The adjustment occurs in a reversible, rapid, and robust manner (R3 rule).
-
Reversibility:
-
The chemical or physico-chemical process to interconvert the different states of the catalyst needs to be able to operate readily in both directions under practical conditions.
-
Rapidity:
-
The chemical or physico-chemical process to interconvert from one state to another must occur at a reasonable rate compatible with the time-line of production. Operating windows in the range of seconds to an hour would seem compatible with most conceivable applications.
-
Robustness:
-
The chemical or physico-chemical process to interconvert the different states of the catalyst must not result in an increased rate of catalyst deactivation or in accumulation of undesired by-products as this would lead to a “dead end” after only few cycles. This also implies that the adjustment can be initiated without major modification to the configuration of the equipment.
A central element of adaptive catalytic systems is the response to an external stimulus or trigger resulting in a controlled and reversible change between two (or even more) states (Figure 1b). If the stimulus turns catalytic activity ON or OFF in a rapid and reversible manner, the catalytic system can be stopped or started adaptively reacting for example in real time to intermittent energy supply. If the two states are characterized by distinct selectivities, a given substrate can be converted into products of different value or different market sizes.
A typical example is the catalytic conversion of CO2 to the formate, formaldehyde, or methanol reduction levels as entry points to a range of value-added products.6 Selectively converting individual components from substrate mixtures can also be envisaged to be triggered in such a way. The concept thus opens intriguing opportunities to cope with external dynamics resulting from energy supply or quality variations in chemical feedstock, or to enable customized production.
While catalysts falling into the adaptive category have been reported in the past, the individual cases remain isolated examples or circumstantial observations. Upon a more systematic analysis, strategic approaches are emerging to develop catalytic systems with switchable reactivity through the application of various external stimuli with temperature, irradiation, or reversible chemical events being mostly applied as trigger.7, 8 Representative examples are discussed below in relationship to the R3 rule of adaptivity.
2 Promising Triggers and Selected Examples
2.1 Temperature
Most obviously, temperature can be used as a highly effective control parameter because it directly impacts on reaction rates even without necessarily changing the structure of the active site of a catalyst. Conventional heating is a relatively slow process, however, as the temperature at the catalyst surface is adjusted through the reactor material and the bulk reaction mixture. In contrast, magnetic induction heating provides an instantaneous and highly controlled way to provide thermal energy input at well-defined confinements.9 For example, magnetically induced adaptive gas phase hydrogenation of CO2 to CH4 was demonstrated under continuous flow conditions using iron carbide nanoparticles (ICNPs) immobilized on SiRAlOx containing small Ru nanoparticles (NPs).9b The catalytic system could be used for 150 h on stream with many start/stop cycles without any decrease in heating power or catalytic performance. Motivated by this opportunity, we developed in collaboration with the Chaudret group multifunctional catalysts integrating active materials with magnetic heating capabilities in order to beneficiate from an extremely localized, rapid, and energy efficient catalyst heating (Figure 2a). ICNPs with excellent specific absorption rates (SAR=heating power) were decorated on the surface of a commercial copper chromite hydrogenation catalyst to demonstrate the general applicability of the method (Figure 2b).10 The multifunctional systems ICNPs@Cu2Cr2O5 could be applied to liquid-phase hydrogenation of proto-typical substrates, responding in real-time to the application of the magnetic field allowing to switch the reaction ON or OFF even under continuous-flow operation (Figure 2c,d). Such immediate response from the catalyst to the magnetic field is of great interest to tackle the industrial challenges associated with intermittent electricity supply. Subsequently, it was demonstrated that inversely catalytically active metal particles can be deposited also on ICNPs or other suitable magnetic NPs potentially allowing for even more direct heat dissipation (e.g., Ni@ICNPs,9b Ru@ICNPs,11 Cu@ICNPs,12 Ni@FeNi313). Demonstrating the potential for adaptive catalysis, multifunctional Cu@ICNPs systems proved highly active for hydrodeoxygenation12 reactions under magnetically induced heating, showing again an immediate response to the magnetic field induced by intermittent electricity supply.
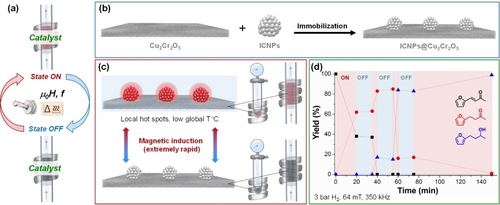
Magnetic induction heating as trigger for rapid, robust, and reversible switching of catalytic activity. Concept (a) exemplified through a recent study on the development of adaptive heterogeneous catalysts with magnetic heating capabilities (b–c) for the selective hydrogenation of aromatic ketones (d).10 ICNPs=iron carbide nanoparticles. μ0H=magnetic field amplitude, f=magnetic field frequency.
Besides magnetic induction, light irradiation offers the possibility to heat appropriately designed plasmonic materials in a rapid and localized manner through localized surface plasmon resonance.14 This photo-thermal effect has been studied in catalysis in particular for the reduction of CO2.15, 16 For example, Zeng et al. reported the preparation of hybrid structures formed by Pt nanocubes (6 nm) and Au nano-cages (31 nm) inside of the well-known ZIF-8 MOF, and their application to the hydrogenation of CO2 to methanol.17 The three-component Pt@Au@ZIF-8 catalytic system showed enhanced methanol productivity (TOF=1500 h−1) under light irradiation as compared to dark conditions (TOF=120 h−1) at identical global temperature of 150 °C. In this case, the authors propose that Au nanocages act as nano-heaters activating the Pt sites responsible for the conversion of CO2. Ye and co-workers reported the synthesis of ultrathin amorphous Y2O3 nanosheets with confined single nickel atoms.18 Associated to a selective light absorber (AlNx/Al foil), the catalytic system reached a temperature of 240 °C under 1 sun irradiation, hydrogenating CO2 selectively to CH4 at a rate of 7.5 Lm−2 h−1. Catalytic activity was shown to respond rapidly and reversibly to the activation and deactivation of the light source. However, it is worth mentioning that there is intense discussion in the literature about the evaluation of the thermal contribution to the overall photocatalytic rate in plasmon-mediated reactions.15, 19
In the past decades, the use of thermo-responsive polymeric structures20 also attracted attention to modulate the activity and selectivity of catalytic systems.21 Indeed, these sometimes called “smart” materials can undergo reversible phase transition in response to an increase or decrease of temperature.20, 21 Thermo-responsive polymers are classified into two families, depending on whether they present a lower critical solution temperature (LCST) or an upper critical solution temperature (UCST).22 LCST and UCST are the respective critical temperature points below and above which the polymer and solvent are completely miscible.
In a pioneering study, Bergbreiter and co-workers showed that cationic rhodium(I) complexes prepared using ligands based on modified block copolymers of alkene oxides (ethylene oxide and propylene oxide) retain the LCST of the ligands.21a The authors applied the resulting catalysts to the aqueous phase hydrogenation of allyl alcohol, and observed fast conversion at 0 °C. Strikingly, heating the reaction media to 40 °C resulted in a phase separation of the catalyst and a shutdown of catalytic activity. This process was found to be reversible, and catalytic activity could be turned ON or OFF simply by cooling or heating the reaction, respectively. It was proposed that this effect could be exploited for self-controlled reaction temperatures of highly exothermic processes.
As a recent example for the control of two orthogonal catalytic reactions, Liu et al. reported the preparation of a chiral Ru molecular catalyst entrapped in mesoporous silica and coated with a thermo-responsive polymer containing an achiral Pd complex.8a The resulting bifunctional catalyst was applied to the cascade Suzuki coupling/asymmetric transfer hydrogenation of iodoacetophenones and aryl boronic acid, where the two different types of active sites could be activated separately. At 60 °C (T>LSCT), the polymer is collapsed and only Pd-catalyzed coupling takes place. Decreasing the temperature to 15 °C lead to the swelling of the polymer coating, providing access to the Ru catalyst for transfer hydrogenation of the primary product.
While such catalytic systems can be considered as adaptive, the general strategy relies on global changes of reactor temperature, and the switches are typically slower than when using magnetic induction heating or light irradiation.
2.2 Chemical Reactions
While rapid temperature changes influence the process outcome through the differences in activation energy of the envisaged reaction pathways, chemical modification of the active site at the catalyst impacts directly on intermediates and transition states of the catalytic cycle potentially opening or closing certain pathways.
Very recently, Reek and co-workers reported the preparation and application of Pt2L4 cages for the catalytic cyclization of alkynoic acids.23 The authors could finely modulate the activity of their Pt catalyst through the encapsulation in the cages of small molecules that they call effectors and that can interact with the cavity and the metal sites. In particular, they observed a 19-fold increase of alkynoic acid lactonization activity through the addition of maleic acid (50 equiv), and a 5-fold decrease of activity through the addition of dicyanobenzene (50 equiv). However, effector accumulation in the cages can be limiting, and reversibility was not explored in this study. The same group studied hexameric undecyl-resorcin[4]arene capsules, and showed that they can incorporate either 8 or 15 water molecules into their hydrogen-bonded networks. Interestingly, it was found that the capsules containing 8 water molecules are poorly active in a model Diels–Alder cyclization, while the capsule containing 15 water molecules enhanced the catalytic rate by 10-fold.24 Thus, the authors were capable of turning catalytic activity ON and OFF simply by controlling the water content in the reaction media.
Changes in pH through acid/base equilibria have been used in various ways to control catalytic reactions.25 For example, Joó and co-workers have shown that the selectivity of [HRuCl(tppms)3] and [H2Ru(tppms)4] (tppms=(3-sulfonatophenyl)diphenylphosphane) in the hydrogenation of cinnamaldehyde with H2 can be controlled by adjusting the pH value.25a At pH 9, the C=O is hydrogenated and cinnamyl alcohol is obtained, while at pH 3 the selectivity is inverted and dihydrocinnamaldehyde is produced through C=C hydrogenation. More recently, Fan et al. reported a pH responsive Rh molecular complex based on a [2]rotaxane with a phosphine ligand. The authors could show that the movements of the rotaxane wheels could be controlled by addition of acid and base, resulting in the possibility to reversibly switch ON or OFF catalytic activity in the hydrogenation of α,β-dehydroamino acid esters and aryl enamides.26
In general, however, pH-triggered selectivity or activity switches are limited by salt accumulation jeopardizing in longer term robustness as one of the criteria for adaptivity.
In order to fulfil the R3-rule of adaptive catalytic systems, full reversibility of the molecular modification must be achieved. In this context, interactions with carbon dioxide (CO2) are particularly attractive. In analogy to the formation of carbonic acid and carbonates through reaction with water, CO2 reacts reversibly with many nucleophiles including alcohols and amines to bicarbonates and carbamates, respectively. The concept of “CO2 switchable separation” based on this chemistry has been put forward by Eckert and Jessop27 and a range of innovative applications has been developed in particular in the laboratories of the Jessop group.28 Notably, also the catalytic hydrogenation of CO2 to formic acid and formates is long known to be fully reversible6a, 29 as widely discussed more recently in the context of hydrogen storage.30
In order to explore the reversible reactivity of CO2 in catalysis, we combined ruthenium NPs with a CO2-responsive support to rationally design a hydrogenation catalyst capable of adapting its selectivity to the feed gas composition H2 vs H2/CO2.31 Such a catalyst would be able to “recognize” whether the feed gas is composed of pure H2 (e.g., from water electrolysis) or a mixture of H2 with CO2 (e.g., from biomass reforming). Also, introducing CO2 into the feed gas deliberately could be used to “switch” the product formation by reversible selectivity control. Based on previous experience, we deposited ruthenium NPs on an amine-functionalized support in collaboration with the group of Prof. P. Jessop at Queen's University in Kingston, Canada. The amine groups of the support were chosen initially due to their interaction with CO2 to form carbamates or bicarbonates. Spectroscopic investigations revealed that the presence of the ruthenium NPs led to hydrogenation of CO2 generating formate species on the surface, however. The associated molecular changes at the surface were found to be fully reversible, as the ammonium formate decomposes back to H2 and CO2, regenerating the initial amine structure when the CO2 atmosphere was removed. The catalytic performance of the materials in the amine state and the formate state differed drastically. It was verified that depending only on the composition of the feed gas, the catalyst operates in two different modes producing selectively two different products from the same starting material under otherwise identical conditions. In particular, when the hydrogenation was performed under a gas stream of pure H2, biomass-derived furfuralacetone was completely hydrogenated to give the corresponding saturated alcohol. However, when a mixture of H2 and CO2 was used, a sharp change in selectivity was observed, where the furan ring and the double bond were still hydrogenated while the ketone was conserved. This adaptive change in selectivity could be applied to a range of furan derivatives of different structures. Notably, the two modes of operation could be alternated by switching ON or OFF the CO2 supply to the gas stream in almost in real time (Figure 3). Such selectivity control is particularly interesting to enable flexible and customized production schemes on the basis of renewable feedstocks.
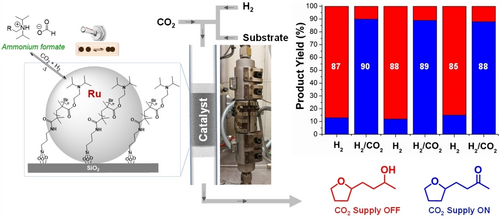
Switchable hydrogenation of biomass-derived furfuralacetone to two different products using a CO2-responsive adaptive catalytic system.31
2.3 Photochemical Irradiation
Natural photoresponsive processes such as photosynthesis and vision show how suitable is light in initiating and regulating complex molecular and biochemical processes. As a result, the use of light to control the activity and selectivity of catalysts in a reversible manner attracted tremendous attention, as it is a non-invasive method with the potential of precise regulation.7a, 32 The structure of photoadaptive catalytic systems typically contains photoactive units in close interaction with the catalytically active sites. Following the pioneering work from Feringa et al. on light driven molecular rotors,33 the light-induced cis-trans isomerization of double bonds attracted a lot of attention as robust, rapid, and reversible photoswitch. Current examples for photoadaptive catalytic systems include in particular the cis-trans isomerization of azobenzene units to control the reactivity of organocatalysts, metal complexes, and metal NPs.34 It is important to mention that constant irradiation is typically required to maintain the desired conformation with high selectivity. As a selected example, Grzybowski et al. reported the preparation of a catalytic system composed of Au NPs stabilized by azobenzene-thiol ligands whose reactivity can be turned ON or OFF through light-induced aggregation of the Au NPs (Figure 4).34a Under UV irradiation, the ligands undergo a trans-to-cis isomerization, thereby developing 5 D electric dipoles that translate into attractive forces between NPs in non-polar solvent. As a result, poor activity for the hydrosilylation of 4-methoxybenzaldehyde was observed. Turning OFF the UV irradiation and using visible light allows re-isomerization to the trans form of the ligand, shutting down the interparticle attractions and providing individual NPs possessing high catalytic activity. This process was found robust, rapid (a few minutes are sufficient for the switches to occur), and fully reversible.
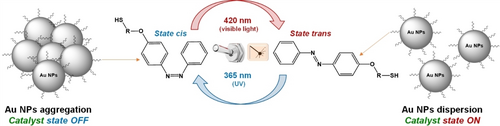
Light-induced cis-trans isomerization of double bonds as trigger to control catalytic performance. Concept exemplified by the reversible dispersion/aggregation (i.e., activation/deactivation) of Au NPs hydrosilylation catalysts through the isomerization of their azobenzene-thiol ligands.34a
The reversible isomerization of double bonds is also a widely applied photo-switch to control the selectivity of catalysts, and in particular their stereoselectivity. For example, Feringa et al. have shown that the light-driven isomerization of a C=C bond in a molecular catalyst allows controlling the catalyst performance with respect to activity and absolute stereocontrol in an asymmetric transformation.34f The chiral product of a Michael addition could be selectively obtained in the S or R configuration by tuning the structure of the organocatalyst through the application of light or temperature. Based on a similar strategy, several groups have reported photoswitchable chirality control in various transformations including for example Heck reaction,35 Pd-catalyzed desymmetrization reaction,36 Steglich rearrangement of O-carboxylazlactones,37 and addition of silyl ketene acetal to 1-chloroisochromans.38
Besides the isomerization of double bonds, other types of photoadaptive catalytic systems have been punctually reported. For example, Chirik and co-workers showed that a Co-based molecular catalyst ((R,R)-(iPrDuPhos)Co(CO)2H) exposed to blue light possesses a much higher activity and tolerance to substrates for the hydrogenation of alkenes than the same catalyst heated to 100 °C.8c The authors attribute this effect to a change in the mechanism and intermediates formed, involving light-induced dissociation of a carbonyl ligand followed by a coordination-insertion sequence where the product is released by combination of a cobalt alkyl intermediate with the starting hydride complex. Recently, Werlé et al. reported a light-induced Fe-based catalytic systems for the reaction of aldehydes with trimethylsilyl azide to produce amides, while a similar Fe-catalyst directed the reaction to nitriles in the dark.39 Although these and other examples highlight the possibility to influence the reactivity especially of 3d metals upon irradiation, the demonstration of reversible switching is rarely reported so far.
2.4 Redox Switches
Redox-active ligands are attracting attention to incorporate stimuli-responsive units in catalytic systems and acquire a level of control over chemical transformations.40 In this context, N-heterocyclic carbenes (NHC) were recently highlighted and qualified as “smart” ligands for their ability to adjust their properties to the requirements of specific catalytic transformations.41 Plenio et al. pioneered the development of NHC ligand-based redox switchable catalysts by reporting a Ru Grubbs-Hoveyda type complex bearing a NHC ligand possessing two ferrocenyl groups acting as redox-responsive units.40b By controlling the solubility of these redox-switchable phase tags using redox reagents or electrochemical redox processes, the authors could effectively and reversibly switch ON and OFF the activity of the olefin metathesis catalyst.
Later, Peris and co-workers reported the preparation of naphthalene-diimide-functionalized complexes of rhodium and iridium that can undergo two sequential one-electron reductions of the ligand.42 These complexes were applied to the catalytic cycloisomerization of alkynoic acids, showing that the one-electron reduction results in a great enhancement of the catalytic activity of the process. Interestingly, the authors could activate or deactivate their catalysts simply by adding a reductant or an oxidant, and suggest that this switching process may be even achievable electrochemically, which would prevent waste accumulation. Using such redox-switchable catalysts for the cycloisomerization of 4-pentynoic acid, the authors reached TON values up to 10 000. Following a comparable strategy, the same authors prepared and characterized a gold complex with a naphthalene-diimide-functionalized N-heterocyclic carbene ligand capable of undergoing two successive reduction events.43 While the neutral complex was found active for the hydroamination of terminal alkynes, the one-electron reduced species did not show any activity. The authors could turn ON and OFF catalytic activity by alternatively introducing cobaltocene or acetylferrocenium tetrafluoroborate, demonstrating the switchability of their catalyst (Figure 5).
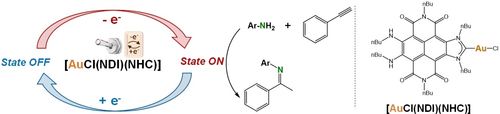
Redox switchable activity of an [AuCl(NDI)(NHC)] complex for the hydroamination of terminal alkynes.43
These selected examples highlight the potential of redox triggers to control and switch the activity of appropriately designed catalysts. While in most of the cases the switches are produced through the addition of reductants or oxidants, the potential to use directly electrochemical processes is there.
3 Conclusion and Outlook
While the field of adaptive catalysis is still in its infancy, it holds a promising potential for chemical energy conversion in science and application. The examples collected here comprise only the tip of an iceberg of opportunities, as the concept is by no means limited to specific reaction types. Processes involving hydrogen activation and transfer seem particularly attractive to open new opportunities for innovative production schemes on basis of renewable feedstock and energy supply. In practical terms, this relates to the challenges of intermittent energy supply and feedstock variations. On the molecular level, the control of H2 activation from homolytic to heterolytic is a decisive factor for chemoselectivity, and changes in spatial arrangements at the active site allow control over the stereochemical outcome. The fundamental principles to design, prepare, and control catalysts and catalytic reactions in a truly adaptive manner remain as yet poorly developed, but certain trends and guidelines are emerging. The present article is hoped to promote this scientific concept, to stimulate an active research area, and to contribute to a critical discussion about the potential and limitations of dynamic processing schemes for a post-fossil chemical value chain.
Disclaimer
The opinions expressed in this publication are the views of the authors and do not necessarily reflect the opinions or views of Angewandte Chemie International Edition/Angewandte Chemie, the Publisher, the GDCh, or the affiliated editors.
Acknowledgments
The authors acknowledge financial support by the Max Planck Society and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany′s Excellence Strategy—Exzellenzcluster 2186 “The Fuel Science Center” ID: 390919832. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.