Hemin as a General Static Dark Quencher for Constructing Heme Oxygenase-1 Fluorescent Probes
Fan Chen
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Baoxin Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhenjiang Ding
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Current address: Beijing Key Laboratory of the Innovative Development of Functional Staple and Nutritional Intervention for Chronic Diseases, China National Research Institute of Food and Fermentation Industries, Beijing, 100015 China
Search for more papers by this authorMiao Zhong
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorDr. Yanan Hou
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorFang Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorDr. Guodong Hu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Jianguo Fang
School of Chemistry and Chemical Engineering, Nanjing University of Science & Technology, Nanjing, Jiangsu, 210094 China
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorFan Chen
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Baoxin Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhenjiang Ding
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Current address: Beijing Key Laboratory of the Innovative Development of Functional Staple and Nutritional Intervention for Chronic Diseases, China National Research Institute of Food and Fermentation Industries, Beijing, 100015 China
Search for more papers by this authorMiao Zhong
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorDr. Yanan Hou
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorFang Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorDr. Guodong Hu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorCorresponding Author
Prof. Jianguo Fang
School of Chemistry and Chemical Engineering, Nanjing University of Science & Technology, Nanjing, Jiangsu, 210094 China
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorGraphical Abstract
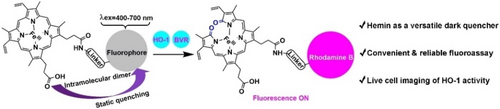
Hemin was identified as a broad-spectrum (400–700 nm) and highly efficient (quenching efficiency >97 %) dark quencher for designing “turn-on” fluorescent probes. Based on this initial discovery, the first live cell-applicable fluorescent probe of heme oxygenase-1 (HO-1) was disclosed and a convenient fluoroassay was developed for determining HO-1 activity in biological samples.
Abstract
The development of small-molecule probes suitable for live-cell applications remains challenging yet highly desirable. We report the first fluorescent probe, RBH, for imaging the heme oxygenase-1 (HO-1) activity in live cells after discovering hemin as a universal dark quencher. Hemin works via a static quenching mechanism and shows high quenching efficiency (>97 %) with fluorophores across a broad spectrum (λex=400–700 nm). The favorable properties of RBH (e.g. long excitation/emission wavelengths, fast response rate and high magnitude of signal increase) enable its use for determining HO-1 activity in complex biological samples. As HO-1 is involved in regulating antioxidant defence, iron homeostasis and gasotransmitter carbon monoxide production, we expect RBH to be a powerful tool for dissecting its functions. Also, the discovery of hemin as a general static dark quencher provides a straightforward strategy for constructing novel fluorescent probes for diverse biological species.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202301598-sup-0001-misc_information.pdf4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Myochin, K. Hanaoka, S. Iwaki, T. Ueno, T. Komatsu, T. Terai, T. Nagano, Y. Urano, J. Am. Chem. Soc. 2015, 137, 4759–4765;
- 1bA. Chevalier, J. Hardouin, P. Y. Renard, A. Romieu, Org. Lett. 2013, 15, 6082–6085;
- 1cA. Chevalier, P. Y. Renard, A. Romieu, Org. Lett. 2014, 16, 3946–3949;
- 1dB. Fang, Y. Shen, B. Peng, H. Bai, L. Wang, J. Zhang, W. Hu, L. Fu, W. Zhang, L. Li, W. Huang, Angew. Chem. Int. Ed. 2022, 61, e202207188;
- 1eT. C. Alich, M. Pabst, L. Pothmann, B. Szalontai, G. C. Faas, I. Mody, Proc. Natl. Acad. Sci. USA 2021, 118, e2020235118;
- 1fY. Wen, N. Jing, F. Huo, C. Yin, Chin. Chem. Lett. 2023, 34, 107604.
- 2
- 2aL. Le Reste, J. Hohlbein, K. Gryte, A. N. Kapanidis, Biophys. J. 2012, 102, 2658–2668;
- 2bM. K. Johansson, R. M. Cook, Chem. Eur. J. 2003, 9, 3466–3471.
- 3
- 3aR. B. Sekar, A. Periasamy, J. Cell Biol. 2003, 160, 629–633;
- 3bL. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler, T. D. James, Chem. Soc. Rev. 2020, 49, 5110–5139.
- 4
- 4aM. Rahman, H. J. Harmon, Spectrochim. Acta Part A 2006, 65, 901–906;
- 4bJ. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, New York, 2006;
- 4cM. K. Johansson, H. Fidder, D. Dick, R. M. Cook, J. Am. Chem. Soc. 2002, 124, 6950–6956.
- 5
- 5aA. Chevalier, P. Y. Renard, A. Romieu, Chem. Asian J. 2017, 12, 2008–2028;
- 5bE. D. Matayoshi, G. T. Wang, G. A. Krafft, J. Erickson, Science 1990, 247, 954–958.
- 6S. Takahashi, W. Piao, Y. Matsumura, T. Komatsu, T. Ueno, T. Terai, T. Kamachi, M. Kohno, T. Nagano, K. Hanaoka, J. Am. Chem. Soc. 2012, 134, 19588–19591.
- 7X. Peng, H. Chen, D. R. Draney, W. Volcheck, A. Schutz-Geschwender, D. M. Olive, Anal. Biochem. 2009, 388, 220–228.
- 8
- 8aQ. Wang, L. Yu, R. C. H. Wong, P. C. Lo, Eur. J. Med. Chem. 2019, 179, 828–836;
- 8bJ. Mu, F. Liu, M. S. Rajab, M. Shi, S. Li, C. Goh, L. Lu, Q. H. Xu, B. Liu, L. G. Ng, B. Xing, Angew. Chem. Int. Ed. 2014, 53, 14357–14362.
- 9P. M. Holland, R. D. Abramson, R. Watson, D. H. Gelfand, Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280.
- 10
- 10aA. Rudkouskaya, N. Sinsuebphon, M. Ochoa, S. J. Chen, J. E. Mazurkiewicz, X. Intes, M. Barroso, Theranostics 2020, 10, 10309–10325;
- 10bA. K. Rudd, N. Mittal, E. W. Lim, C. M. Metallo, N. K. Devaraj, J. Am. Chem. Soc. 2020, 142, 17887–17891.
- 11P. Crisalli, E. T. Kool, Bioconjugate Chem. 2011, 22, 2345–2354.
- 12J. Demuth, R. Kucera, K. Kopecky, Z. Havlinova, A. Libra, V. Novakova, M. Miletin, P. Zimcik, Chem. Eur. J. 2018, 24, 9658–9666.
- 13J. Wu, Y. Tan, Y. Xie, Y. Wu, R. Zhao, Y. Jiang, C. Tan, Chem. Commun. 2013, 49, 11379–11381.
- 14
- 14aD. Genovese, M. Cingolani, E. Rampazzo, L. Prodi, N. Zaccheroni, Chem. Soc. Rev. 2021, 50, 8414–8427;
- 14bF. E. Jernigan, D. S. Lawrence, Chem. Commun. 2013, 49, 6728–6730.
- 15R. Gozzelino, V. Jeney, M. P. Soares, Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354.
- 16
- 16aK. Krukowska, M. Magierowski, Biochem. Pharmacol. 2022, 201, 115058;
- 16bL. Salerno, G. Romeo, M. N. Modica, E. Amata, V. Sorrenti, I. Barbagallo, V. Pittala, Eur. J. Med. Chem. 2017, 142, 163–178.
- 17
- 17aV. Vijayan, F. Wagener, S. Immenschuh, Biochem. Pharmacol. 2018, 153, 159–167;
- 17bA. Paine, B. Eiz-Vesper, R. Blasczyk, S. Immenschuh, Biochem. Pharmacol. 2010, 80, 1895–1903.
- 18
- 18aL. Bellner, N. B. Lebovics, R. Rubinstein, Y. D. Buchen, E. Sinatra, G. Sinatra, N. G. Abraham, J. A. McClung, E. A. Thompson, Antioxid. Redox Signaling 2020, 32, 1045–1060;
- 18bM. L. Wu, Y. C. Ho, S. F. Yet, Antioxid. Redox Signaling 2011, 15, 1835–1846.
- 19
- 19aZ. Si, X. Wang, J. Alzheimer′s Dis. 2020, 78, 1259–1272;
- 19bD. D. Haines, M. V. Trushin, S. Rose, I. A. S. Bernard, F. F. Mahmoud, Curr. Pharm. Des. 2018, 24, 2317–2321.
- 20
- 20aS. Ryter, E. Kvam, R. M. Tyrrell, Methods Enzymol. 1999, 300, 322–336;
- 20bB. A. Schacter, Methods Enzymol. 1978, 52, 367–372.
- 21H. J. Vreman, D. K. Stevenson, Anal. Biochem. 1988, 168, 31–38.
- 22R. K. Kutty, M. D. Maines, J. Biol. Chem. 1981, 256, 3956–3962.
- 23R. Tenhunen, H. S. Marver, R. Schmid, J. Biol. Chem. 1969, 244, 6388–6394.
- 24M. D. Maines, A. Kappas, Proc. Natl. Acad. Sci. USA 1974, 71, 4293–4297.
- 25
- 25aY. Hou, J. Li, J. C. Wu, Q. X. Wu, J. Fang, ACS Chem. Neurosci. 2021, 12, 2798–2809;
- 25bZ. L. Song, F. Bai, B. Zhang, J. Fang, J. Agric. Food Chem. 2020, 68, 2214–2231;
- 25cY. Hou, X. Li, S. Peng, J. Yao, F. Bai, J. Fang, J. Agric. Food Chem. 2019, 67, 8227–8234.
- 26E. R. H. Walter, Y. Ge, J. C. Mason, J. J. Boyle, N. J. Long, J. Am. Chem. Soc. 2021, 143, 6460–6469.
- 27
- 27aX. Wu, R. Wang, N. Kwon, H. Ma, J. Yoon, Chem. Soc. Rev. 2022, 51, 450–463;
- 27bP. Wang, L. Yu, J. Gong, J. Xiong, S. Zi, H. Xie, F. Zhang, Z. Mao, Z. Liu, J. S. Kim, Angew. Chem. Int. Ed. 2022, 61, e202206894;
- 27cX. Wu, R. Wang, S. Qi, N. Kwon, J. Han, H. Kim, H. Li, F. Yu, J. Yoon, Angew. Chem. Int. Ed. 2021, 60, 15418–15425;
- 27dS. Wang, W. X. Ren, J. T. Hou, M. Won, J. An, X. Chen, J. Shu, J. S. Kim, Chem. Soc. Rev. 2021, 50, 8887–8902;
- 27eH. Zhang, L. Shi, K. Li, X. Liu, M. Won, Y. Z. Liu, Y. Choe, X. Y. Liu, Y. H. Liu, S. Y. Chen, K. K. Yu, J. S. Kim, X. Q. Yu, Angew. Chem. Int. Ed. 2022, 61, e202116439;
- 27fJ. Liu, W. Zhang, C. Zhou, M. Li, X. Wang, W. Zhang, Z. Liu, L. Wu, T. D. James, P. Li, B. Tang, J. Am. Chem. Soc. 2022, 144, 13586–13599;
- 27gY. Yao, Y. Zhang, C. Yan, W. H. Zhu, Z. Guo, Chem. Sci. 2021, 12, 9885–9894;
- 27hC. Yan, Z. Guo, W. Chi, W. Fu, S. A. A. Abedi, X. Liu, H. Tian, W. H. Zhu, Nat. Commun. 2021, 12, 3869;
- 27iL. Wu, J. Liu, X. Tian, R. R. Groleau, B. Feng, Y. Yang, A. C. Sedgwick, H. H. Han, Y. Wang, H. M. Wang, F. Huang, S. D. Bull, H. Zhang, C. Huang, Y. Zang, J. Li, X. P. He, P. Li, B. Tang, T. D. James, J. L. Sessler, J. Am. Chem. Soc. 2022, 144, 174–183;
- 27jY. Wang, S. Xu, M. Xian, Chem. Eur. J. 2020, 26, 11673–11683;
- 27kW. Hu, T. Qiang, L. Chai, T. Liang, L. Ren, F. Cheng, C. Li, T. D. James, Chem. Sci. 2022, 13, 5363–5373.
- 28
- 28aG. Hu, M. Zhong, J. Zhao, H. Gao, L. Gan, H. Zhang, S. Zhang, J. Fang, ACS Sens. 2021, 6, 1384–1391;
- 28bG. Hu, H. Jia, Y. Hou, X. Han, L. Gan, J. Si, D. H. Cho, H. Zhang, J. Fang, Anal. Chem. 2020, 92, 4371–4378;
- 28cX. Li, B. Zhang, C. Yan, J. Li, S. Wang, X. Wei, X. Jiang, P. Zhou, J. Fang, Nat. Commun. 2019, 10, 2745;
- 28dL. Zhang, S. Peng, J. Sun, J. Yao, J. Kang, Y. Hu, J. Fang, Chem. Sci. 2017, 8, 2966–2972;
- 28eB. Zhang, C. Ge, J. Yao, Y. Liu, H. Xie, J. Fang, J. Am. Chem. Soc. 2015, 137, 757–769;
- 28fL. Zhang, D. Duan, Y. Liu, C. Ge, X. Cui, J. Sun, J. Fang, J. Am. Chem. Soc. 2014, 136, 226–233.
- 29E. Sitte, M. O. Senge, Eur. J. Org. Chem. 2020, 3171–3191.
- 30
- 30aS. Mondal, N. Ghorai, S. Bhunia, H. N. Ghosh, N. Amdursky, Chem. Sci. 2021, 12, 8731–8739;
- 30bL. D. Newton, S. I. Pascu, R. M. Tyrrell, I. M. Eggleston, Org. Biomol. Chem. 2019, 17, 467–471.
- 31A. Osama, J. Zhang, J. Yao, X. Yao, J. Fang, Ageing Res. Rev. 2020, 64, 101206.
- 32H. Jia, G. Hu, D. Shi, L. Gan, H. Zhang, X. Yao, J. Fang, Anal. Chem. 2019, 91, 8524–8531.