Lewis-Pairing-Induced Electrochemiluminescence Enhancement from Electron Donor-Acceptor Diads Decorated with Tris(pentafluorophenyl)borane as an Electrochemical Protector
Takashi Ikeda
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorCorresponding Author
Dr. Keishiro Tahara
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Present address: Faculty of Engineering and Design, Kagawa University, 2217-20 Hayashi-cho, Takamatsu, Kagawa, 761-0396 Japan
Search for more papers by this authorDr. Ryoichi Ishimatsu
Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorProf. Toshikazu Ono
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorDr. Luxia Cui
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorMomoka Maeda
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorProf. Yoshiki Ozawa
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorCorresponding Author
Prof. Masaaki Abe
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorTakashi Ikeda
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorCorresponding Author
Dr. Keishiro Tahara
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Present address: Faculty of Engineering and Design, Kagawa University, 2217-20 Hayashi-cho, Takamatsu, Kagawa, 761-0396 Japan
Search for more papers by this authorDr. Ryoichi Ishimatsu
Department of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorProf. Toshikazu Ono
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorDr. Luxia Cui
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorMomoka Maeda
Department of Chemistry and Biochemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395 Japan
Search for more papers by this authorProf. Yoshiki Ozawa
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorCorresponding Author
Prof. Masaaki Abe
Department of Material Science, Graduate School of Science, University of Hyogo, 3-2-1, Kouto, Kamigori, Ako, Hyogo, 678-1297 Japan
Search for more papers by this authorGraphical Abstract
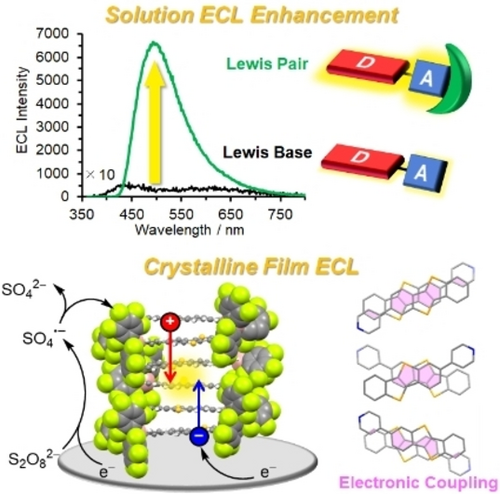
The feasibility of electrochemical protectors was demonstrated with a classical Lewis acid B(C6F5)3, which was mounted on donor-acceptor emitters to greatly stabilize electrogenerated radicals and enhance the Lewis-pairing-induced electrochemiluminescence (ECL). Electrochemical doping in segregated emitter-B(C6F5)3 co-assemblies afforded a crystalline film showing ECL through singlet exciton delocalization derived from columnar π-stacks.
Abstract
This study reports an effective peripheral decoration of organic donor-acceptor diads with B(C6F5)3 for stabilizing electrogenerated radical ions. By employing a common p-type organic semiconductor benzothienobenzothiophene (BTBT) as the donor, tetracoordinate boron complexes showed improved solution electrochemiluminescence (ECL) intensity, reaching a 156-fold increase compared to that of the parent diad. The unprecedented Lewis-pairing-induced ECL enhancement is attributed to the multiple roles of B(C6F5)3: 1) redistributing frontier orbitals, 2) facilitating electrochemical excitation, and 3) restricting molecular motions. Furthermore, B(C6F5)3 converted the molecular arrangement of BTBT from conventional 2D herringbones into 1D π-stacks. This robust, highly ordered columnar nanostructure allowed red-shifting of the crystalline film ECL with electrochemical doping through the electronic coupling pathways of BTBT. Our approach will facilitate the development of elaborate metal-free ECL systems.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202301109-sup-0001-misc_information.pdf3.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. M. Richter, Chem. Rev. 2004, 104, 3003–3036;
- 1bZ. Zhang, P. Du, G. Pu, L. Wei, Y. Wu, J. Guo, X. Lu, Mater. Chem. Front. 2019, 3, 2246–2257;
- 1cM. Hesari, Z. Ding, Acc. Chem. Res. 2017, 50, 218–230;
- 1dY. Zhao, L. Bouffier, G. Xu, G. Loget, N. Sojic, Chem. Sci. 2022, 13, 2528–2550.
- 2
- 2aW. Miao, Chem. Rev. 2008, 108, 2506–2553;
- 2bZ. Liu, W. Qi, G. Xu, Chem. Soc. Rev. 2015, 44, 3117–3142;
- 2cK. Hiramoto, E. Villani, T. Iwama, K. Komatsu, S. Inagi, K. Y. Inoue, Y. Nashimoto, K. Ino, H. Shiku, Micromachines 2020, 11, 530.
- 3
- 3aN. Kobayashi, T. Kasahara, T. Edura, J. Oshima, R. Ishimatsu, M. Tsuwaki, T. Imato, S. Shoji, J. Mizuno, Sci. Rep. 2015, 5, 14822;
- 3bS. H. Kong, J. I. Lee, S. Kim, M. S. Kang, ACS Photonics 2018, 5, 267–277;
- 3cK. G. Cho, J. I. Lee, S. Lee, K. Hong, M. S. Kang, K. H. Lee, Adv. Funct. Mater. 2020, 30, 1907936.
- 4
- 4aW. Miao, J.-P. Choi, A. J. Bard, J. Am. Chem. Soc. 2002, 124, 14478–14485;
- 4bW. R. Kitzmann, K. Heinze, Angew. Chem. Int. Ed. 2023, 62, e202213207; Angew. Chem. 2023, 135, e202213207.
- 5W. Guo, H. Ding, C. Gu, Y. Liu, X. Jiang, B. Su, Y. Shao, J. Am. Chem. Soc. 2018, 140, 15904–15915.
- 6S. Carrara, A. Aliprandi, C. F. Hogan, L. De Cola, J. Am. Chem. Soc. 2017, 139, 14605–14610.
- 7
- 7aJ.-L. Liu, J.-Q. Zhang, Z.-L. Tang, Y. Zhuo, Y.-Q. Chai, R. Yuan, Chem. Sci. 2019, 10, 4497–4501;
- 7bZ. Han, Z. Yang, H. Sun, Y. Xu, X. Ma, D. Shan, J. Chen, S. Huo, Z. Zhang, P. Du, X. Lu, Angew. Chem. Int. Ed. 2019, 58, 5915–5919; Angew. Chem. 2019, 131, 5976–5980;
- 7cY. Zhang, Y. Zhao, Z. Han, R. Zhang, P. Du, Y. Wu, X. Lu, Angew. Chem. Int. Ed. 2020, 59, 23261–23267; Angew. Chem. 2020, 132, 23461–23467;
- 7dL. Yang, D. Koo, J. Wu, J. M. Wong, T. Day, R. Zhang, H. Kolongoda, K. Liu, J. Wang, Z. Ding, B. L. Pagenkopf, Chem. Eur. J. 2020, 26, 11715–11721;
- 7eJ. M. Wong, R. Zhang, P. Xie, L. Yang, M. Zhang, R. Zhou, R. Wang, Y. Shen, B. Yang, H. B. Wang, Z. Ding, Angew. Chem. Int. Ed. 2020, 59, 17461–17466; Angew. Chem. 2020, 132, 17614–17619;
- 7fG. Liao, J. Zhang, X. Zheng, X. Jia, J. Xu, F. Zhao, N. Wang, K. Liu, P. Chen, X. Yin, Chem. Commun. 2021, 57, 7926–7929.
- 8R. R. Maar, R. Zhang, D. G. Stephens, Z. Ding, J. B. Gilroy, Angew. Chem. Int. Ed. 2019, 58, 1052–1056; Angew. Chem. 2019, 131, 1064–1068.
- 9F. Zinna, S. Voci, L. Arrico, E. Brun, A. Homberg, L. Bouffier, T. Funaioli, J. Lacour, N. Sojic, L. Di Bari, Angew. Chem. Int. Ed. 2019, 58, 6952–6956; Angew. Chem. 2019, 131, 7026–7030.
- 10
- 10aL. R. Faulkner, A. J. Bard, J. Am. Chem. Soc. 1968, 90, 6284–6290;
- 10bH. Yang, A. J. Bard, J. Electroanal. Chem. Interfacial Electrochem. 1991, 306, 87–109.
- 11
- 11aJ.-W. Oh, Y. O. Lee, T. H. Kim, K. C. Ko, J. Y. Lee, H. Kim, J. S. Kim, Angew. Chem. Int. Ed. 2009, 48, 2522–2524; Angew. Chem. 2009, 121, 2560–2562;
- 11bY. O. Lee, T. Pradhan, S. Yoo, T. H. Kim, J. Kim, J. S. Kim, J. Org. Chem. 2012, 77, 11007–11013;
- 11cK. M. Omer, S. Y. Ku, K. T. Wong, A. J. Bard, Angew. Chem. Int. Ed. 2009, 48, 9300–9303; Angew. Chem. 2009, 121, 9464–9467;
- 11dH. Qi, Y.-H. Chen, C.-H. Cheng, A. J. Bard, J. Am. Chem. Soc. 2013, 135, 9041–9049.
- 12H. Imahori, Y. Kobori, H. Kaji, Acc. Mater. Res. 2021, 2, 501–514.
- 13
- 13aX. Tian, L. C. Murfin, L. Wu, S. E. Lewis, T. D. James, Chem. Sci. 2021, 12, 3406–3426;
- 13bA. S. Klymchenko, Acc. Chem. Res. 2017, 50, 366–375.
- 14N. Li, F. Ni, X. Lv, Z. Huang, X. Cao, C. Yang, Adv. Opt. Mater. 2022, 10, 2101343.
- 15
- 15aM. Madhu, R. Ramakrishnan, V. Vijay, M. Hariharan, Chem. Rev. 2021, 121, 8234–8284;
- 15bG. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K.-Y. Jen, H.-L. Yip, Chem. Rev. 2022, 122, 14180–14274.
- 16
- 16aN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075–10166;
- 16bA.-H. Bonardi, F. Dumur, G. Noirbent, J. Lalevée, D. Gigmes, Beilstein J. Org. Chem. 2018, 14, 3025–3046.
- 17A. Kapturkiewicz, ChemElectroChem 2017, 4, 1604–1638.
- 18H. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature 2012, 492, 234–238.
- 19
- 19aH. Liu, L. Wang, H. Gao, H. Qi, Q. Gao, C. Zhang, ACS Appl. Mater. Interfaces 2017, 9, 44324–44331;
- 19bX. Wei, M.-J. Zhu, Z. Cheng, M. Lee, H. Yan, C. Lu, J.-J. Xu, Angew. Chem. Int. Ed. 2019, 58, 3162–3166; Angew. Chem. 2019, 131, 3194–3198.
- 20
- 20aR. Ishimatsu, S. Matsunami, T. Kasahara, J. Mizuno, T. Edura, C. Adachi, K. Nakano, T. Imato, Angew. Chem. Int. Ed. 2014, 53, 6993–6996; Angew. Chem. 2014, 126, 7113–7116;
- 20bS. Kumar, P. Tourneur, J. R. Adsetts, M. Y. Wong, P. Rajamalli, D. Chen, R. Lazzaroni, P. Viville, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, J. Cornil, Z. Ding, E. Zysman-Colman, J. Mater. Chem. C 2022, 10, 4646–4667.
- 21J. R. Adsetts, Z. Ding, ChemPlusChem 2021, 86, 155–165.
- 22
- 22aR. Luo, H. Lv, Q. Liao, N. Wang, J. Yang, Y. Li, K. Xi, X. Wu, H. Ju, J. Lei, Nat. Commun. 2021, 12, 6808;
- 22bZ. Jin, X. Zhu, N. Wang, Y. Li, H. Ju, J. Lei, Angew. Chem. Int. Ed. 2020, 59, 10446–10450; Angew. Chem. 2020, 132, 10532–10536.
- 23
- 23aM. M. Hansmann, A. López-Andarias, E. Rettenmeier, C. Egler-Lucas, F. Rominger, A. S. K. Hashmi, C. Romero-Nieto, Angew. Chem. Int. Ed. 2016, 55, 1196–1199; Angew. Chem. 2016, 128, 1212–1216;
- 23bT. Mori, Y. Yoshigoe, Y. Kuninobu, Angew. Chem. Int. Ed. 2019, 58, 14457–14461; Angew. Chem. 2019, 131, 14599–14603;
- 23cJ. Zou, Y. Fang, Y. Shen, Y. Xia, K. Wang, C. Zhang, Y. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202207426; Angew. Chem. 2022, 134, e202207426;
- 23dT. Ono, M. Sugimoto, Y. Hisaeda, J. Am. Chem. Soc. 2015, 137, 9519–9522;
- 23eS. Hatanaka, T. Ono, Y. Yano, D. T. Gryko, Y. Hisaeda, ChemPhotoChem 2020, 4, 138–143.
- 24
- 24aF. Focante, P. Mercandelli, A. Sironi, L. Resconi, Coord. Chem. Rev. 2006, 250, 170–188;
- 24bS. J. Geier, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 3476–3477.
- 25H. Na, A. Maity, T. S. Teets, Organometallics 2016, 35, 2267–2274.
- 26
- 26aT. Hinoue, M. Miyata, I. Hisaki, N. Tohnai, Angew. Chem. Int. Ed. 2012, 51, 155–158; Angew. Chem. 2012, 124, 159–162;
- 26bZ.-Q. Li, Z.-L. Gong, J.-Y. Shao, J. Yao, Y.-W. Zhong, Angew. Chem. Int. Ed. 2021, 60, 14595–14600; Angew. Chem. 2021, 133, 14716–14721.
- 27
- 27aD. Yan, A. Delori, G. O. Lloyd, T. Friščič, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans, X. Duan, Angew. Chem. Int. Ed. 2011, 50, 12483–12486; Angew. Chem. 2011, 123, 12691–12694;
- 27bM. S. Kwon, D. Lee, S. Seo, J. Jung, J. Kim, Angew. Chem. Int. Ed. 2014, 53, 11177–11181; Angew. Chem. 2014, 126, 11359–11363.
- 28X. Liu, J. M. Cole, P. G. Waddell, T.-C. Lin, J. Radia, A. Zeidler, J. Phys. Chem. A 2012, 116, 727–737.
- 29
- 29aK. Takimiya, H. Ebata, K. Sakamoto, T. Izawa, T. Otsubo, Y. Kunug, J. Am. Chem. Soc. 2006, 128, 12604–12605;
- 29bH. Minemawari, Y. Toshikazu, H. Matui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai, T. Hasegawa, Nature 2011, 475, 364–367.
- 30
- 30aR. Akai, K. Oka, S. Dekura, H. Mori, N. Tohnai, Bull. Chem. Soc. Jpn. 2022, 95, 1178–1182;
- 30bR. Ozdemir, K. Ahn, İ. Deneme, Y. Zorlu, D. Kim, M.-G. Kim, H. Usta, J. Mater. Chem. C 2020, 8, 15253–15267.
- 31
- 31aE. Lippert, Z. Naturforsch. A 1955, 10, 541–545;
- 31bN. Mataga, Y. Kaifu, M. Koizumi, Bull. Chem. Soc. Jpn. 1955, 28, 690–691.
- 32T. P. Vaid, M. E. Cook, J. D. Scott, M. B. Carazo, J. Ruchti, S. D. Minteer, M. S. Sigman, A. J. McNeil, M. S. Sanford, Chem. Eur. J. 2022, 28, e202202147.
- 33H. Hou, Y. Wang, Y. Wang, R. Luo, D. Zhu, J. Zhou, X. Wu, H. Ju, J. Lei, J. Mater. Chem. C 2022, 10, 14488–14495.
- 34Deposition number 2116421 (for 1 p-TPFB) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.