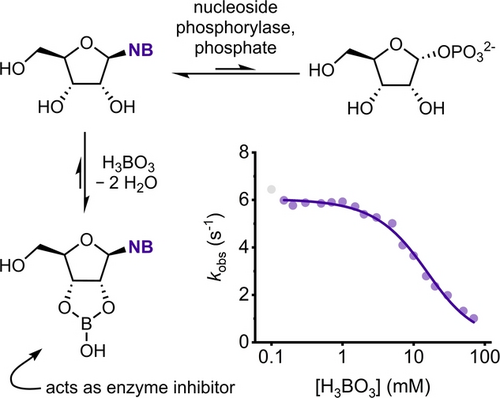
Biased Borate Esterification during Nucleoside Phosphorylase-Catalyzed Reactions: Apparent Equilibrium Shifts and Kinetic Implications**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2022-xjvpw).
Graphical Abstract
Biased esterification of nucleosides with borate preferentially removes those reactants from biocatalytic equilibrium systems, with the resulting cyclic borate esters acting as reversible enzyme inhibitors. This effect can be used to manipulate glycosylation equilibria and facilitate biocatalytic access to nucleoside analogues.
Abstract
Biocatalytic nucleoside (trans-)glycosylations catalyzed by nucleoside phosphorylases have evolved into a practical and convenient approach to the preparation of modified nucleosides, which are important pharmaceuticals for the treatment of various cancers and viral infections. However, the obtained yields in these reactions are generally determined exclusively by the innate thermodynamic properties of the nucleosides involved, hampering the biocatalytic access to many sought-after target nucleosides. We herein report an additional means for reaction engineering of these systems. We show how apparent equilibrium shifts in phosphorolysis and glycosylation reactions can be effected through entropically driven, biased esterification of nucleosides and ribosyl phosphates with inorganic borate. Our multifaceted analysis further describes the kinetic implications of this in situ reactant esterification for a model phosphorylase.
Nucleosides and their analogs are central to the biological and chemical sciences, as they serve a variety of biological functions and represent a growing class of anti-cancer and anti-viral pharmaceuticals.1 Although the synthesis of nucleosides typically proceeds via N-glycosylation of nucleobases with heavily protected sugar synthons, there is an increasing recognition for the inefficiency of the associated synthetic routes.2 Consequently, recent years have experienced renewed interest in the biocatalytic synthesis of natural and modified nucleosides. This includes, for instance, the enzymatic preparation of halogenated purine nucleoside synthons,3 the diversification of alkylated pyrimidine nucleoside analogues,4 the development of high-yielding flow processes,5 and the synthesis of the pharmaceuticals islatravir (anti-HIV)6 and molnupiravir (anti-Covid19)7 in biocatalytic cascades. All these examples employ nucleoside phosphorylases for key (trans-)glycosylation reactions, which enable the installation of ribosyl-based moieties on pyrimidine and purine nucleobases in one step and without the need for any protecting group chemistry.
Nucleoside phosphorylases catalyze the reversible phosphorolysis of nucleosides to the corresponding nucleobases and pentose-1-phosphates (Scheme 1).8 This reactivity can be employed in reverse to transfer the glycosyl moiety from one nucleoside (or a pentose-1-phosphate) to another nucleobase, a reaction typically referred to as a (trans-)glycosylation.9 While such (trans-)glycosylation processes are well established as synthetic tools,10-21 they inherently suffer from thermodynamic limitations, as the final yield of these reactions is dictated solely by the substrate-dependent thermodynamics of the respective (reverse) phosphorolytic steps as well as the employed reaction conditions.9, 22, 23 Although some progress has been made to mitigate or exploit the tight thermodynamic control in these systems (e.g., via (by)product precipitation,24 enzymatic product removal25 or application of recoverable excess reagent26) and irreversible phosphorolysis of 6-oxopurines is well established,10, 27, 28 there currently exists no general method for the direct manipulation of glycosylation equilibria.
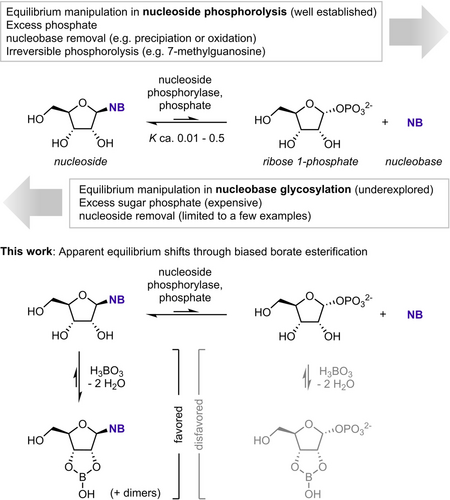
Nucleoside phosphorolysis and strategies for apparent equilibrium shifts. NB=nucleobase.
During our development of the PUB module for continuous high-throughput phosphate detection in biochemical assays,29 we serendipitously found that the presence of borate effects a series of inhibitory phenomena during pyrimidine nucleoside phosphorolysis as well as an apparent equilibrium shift caused by biased borate esterification of nucleosides over ribose 1-phosphate (Rib1P). While saccharides are well known to undergo complex equilibrium reactions with borate in aqueous solution,30, 31 comparably little is known about in situ competition of such processes. Similarly, although the literature offers some examples for biocatalytic applications of equilibrium shift phenomena through preferential esterification of one reactant with borate (namely, the enzymatic epimerization of fructose,32 lactose,33 galactose,34, 35 and arabinose36) the exact species involved in these processes remain largely elusive, as do the kinetic implications of this esterification on the enzyme-level. Despite the precedents for borate inhibiting NAD+-dependent enzymes in a non-competitive fashion,37, 38 the molecular determinants and mechanisms of these phenomena have remained equally elusive. To shed light on the thermodynamic and kinetic implications of borate ester formation on nucleoside phosphorylase-catalyzed reactions and provide a characterized precedent for biased borate esterification, we herein report a multifaceted analysis of this reaction system with spectroscopic and computational approaches. Furthermore, we examined the synthetic utility of this biased borate esterification, as concentration-dependent apparent equilibrium shifts provide an additional means for reaction engineering in biocatalytic glycosylation reactions.
Our investigation was sparked by the serendipitous observation that phosphorolysis reactions with 5-bromouridine (1 a) consistently exhibited noticeably lower equilibrium conversions in the presence of moderate concentrations of borate (Figures 1A and B). For instance, a reaction containing 400 μM 1 a, 4 mM (10 equivalents) phosphate and 40 μg mL−1 (0.45 mol%) of the well characterized pyrimidine nucleoside phosphorylase from Geobacillus thermoglucosidasius (GtPyNP),4, 39-42 serving as a model enzyme (see the Supporting Information for details), reached its equilibrium at 70 % conversion after 10 min in glycine-buffered solution (Figure 1B). In contrast, the same reaction additionally containing 20 mM borate took almost 20 min to reach an equilibrium at 47 % conversion, as monitored by multi-wavelength UV spectroscopy employing principles of spectral unmixing.43, 44 This effect was not rooted in enzyme inactivation, as GtPyNP showed an identical melting point and fully retained its catalytic activity after prolonged incubation in borate-containing buffers (Figures S1 and S2). The observed apparent equilibrium shift persisted in the reverse direction of the reaction (starting from the products Rib1P and 2 a, Figure S6) and additional experiments established that the reaction system consistently behaved according to lower apparent equilibrium constants of phosphorolysis (K′), whose magnitude depended on the concentration of borate (Figure 1C). Since such drastic equilibrium shifts are unprecedented for phosphorolysis systems, we suspected that the formation of a dominant secondary species would actively remove the nucleoside 1 a from this equilibrium in a thermodynamically controlled fashion. Indeed, the conversion data phenotypically following a Boltzmann-type relationship (Figure 1D) could be described well with a thermodynamic model accounting for the presence of an additional equilibrium system in which 1 a is partially transformed to a borate ester (Figure 1E, see the Supporting Information for details and equations). Although borate esters of ribose are known to persist in dilute aqueous solution,45-47 these stable esters generally involve the anomeric hydroxyl group, which is absent in 1 a. Nevertheless, Kim et al.48, 49 observed borate esters of the nucleoside-based cofactor NAD+ and the natural trinucleotides by mass spectrometry, indicating that analogous species might be formed by recruitment of the 2′- and/or 3′-hydroxyl groups. In addition, structurally similar nucleoside boronate esters have been reported by Smietana50, 51 and others.52 Based on these precedents, we hypothesized that the cyclic borate ester 1 a* would be the predominant species causing the observed equilibrium shift (Figure 1F).
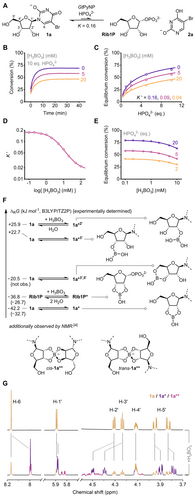
A Phosphorolysis of 5-bromouridine (1 a), and B–E apparent equilibrium shifts observed in the presence of borate. F DFT data. G 1H NMR of 1 a (10 mM) with or without 20 mM borate in 200 mM glycine buffer at pH 9 and 25 °C. See the Supporting Information for experimental details, raw data and equations.58 [a] Not confidently accessible by DFT due to their high charge.
To probe if borate esters like 1 a* would be feasible species to effect apparent equilibrium shifts under dilute aqueous reaction conditions, we turned to density functional theory (DFT) calculations and NMR spectroscopy. DFT calculations (using B3LYP/TZ2P53-56 in combination with a COSMO57 solvent model for water, see the Supporting Information for details) suggested that the formation of the 2′- or 3′-borate monoesters of 1 a would be highly disfavored processes, while the formation of the proposed five-membered cyclic diester 1 a* offers around 40 kJ mol−1 net gain in Gibbs free energy, indicating that this esterification could feasibly proceed against the concentration gradient of water (Figure 1F). Indeed, direct evidence for the presence of 1 a* could be obtained by NMR spectroscopy. When 1 a (10 mM) was incubated with 20 mM borate in glycine buffer at pH 9, a dominant borate-32
containing species in equilibrium with free borate could be observed by 11B NMR (Figure S10), while 1H NMR showed additional signals indicative of a modification of the ribosyl moiety of 1 a (Figure 1G). Furthermore, slight changes in the coupling constants across the sugar ring implied the introduction of ring torsion, consistent with the configurational changes required in 1 a* (Table S4). In addition to this ester, we observed two distinct minor components in the mixture, which exhibited identical coupling constants to 1 a* and existed in similar concentrations (ca. 1 : 1.2 ratio). Based on the well-characterized diastereomeric borate ester dimers of methyl apiose described by Ishii and Ono59 and the report of purine nucleoside solubilization as 2 : 1 complexes with borate by Tsuji and colleagues,60 we tentatively ascribe these species as the cis- and trans-isomers 1 a** (Figures 1G and F). Overall, the Gibbs free energies obtained experimentally by NMR spectroscopy or equilibrium state calculations based on UV data (ΔRG ca. −32 kJ mol−1, see the Supporting Information for details) are comparable to those obtained by DFT calculation. Although an analogous six-membered ester (and potentially its dimers) between the 3′- and 5′-hydroxyl groups would introduce similar configurational changes to those ascribed to 1 a*, DFT calculations revealed that its formation is much less exothermic and consequently disfavored. Similarly, the analogous five-membered borate ester of Rib1P is less favored than 1 a* (which is requisite for the observed equilibrium shift), as supported by DFT and NMR data (Figures 1F and S10). In addition to the lower entropic gain, we expect that steric conflicts between the phosphate group and the borate ester as well as charge repulsion account for the less favored formation of Rib1P* compared to 1 a*. Additionally, we observed no trace of dimers of Rib1P* by NMR, which is likely a result of the highly disfavored formal −5 charge of these species. Consequently, the formation of the borate ester 1 a* should be expected to dominate in direct competition during a phosphorolysis reaction, which we could confirm by subjecting phosphorolysis reaction mixtures with increasing borate concentrations to NMR analysis (Figure S13). Further thermodynamic experiments supported entropic effects as a driving force for this biased esterification. Arrhenius plots of the 1 a–1 a* equilibrium obtained by 1H NMR revealed a drastic temperature-dependence, favoring the presence of the free nucleoside at higher temperatures (Figure S11). In agreement with these observations, Arrhenius plots for the borate esterification derived from equilibrium shifts in the phosphorolysis reaction indicated an approach to an energetic balance between 1 a* and Rib1P* at higher temperatures (Figure S5). Collectively, these results describe 1 a* as the dominant borate ester in this reaction system, responsible for depleting the pool of free nucleoside in dilute aqueous solution at room temperature. Consistent with this conclusion, the 2′-deoxy nucleoside deoxy-1 a, incapable of forming 1 a* or 1 a**, did not show an equilibrium shift during its phosphorolysis in the presence of borate or any reaction with borate discernible by NMR (Figure S15).
Next, we sought to identify the cause of the apparently reduced reaction rates in the presence of borate by enlisting kinetic studies and molecular dynamics (MD) simulations. This effect was especially prominent with borate concentrations greater than 20 mM and led to decreases in the reaction rate of GtPyNP by more than a factor of four, as illustrated in Figure 2D. Since high borate concentrations primarily yield 1 a* in solution (and not the enzymatic substrate 1 a), we initially entertained the hypothesis that this phenomenon was a function of the decreased concentration of the free nucleoside substrate and the associated apparent decrease of affinity. However, the Michaelis–Menten kinetics obtained for 1 a (with phosphate in excess) proved inconsistent with this hypothesis. While a reduction in available substrate concentration should primarily result in an increase in the apparent Michaelis–Menten constant KM, we observed no change in KM but instead a sharp decline of the rate constant under saturating substrate concentrations (kcat, Figure 2B). With saturating concentrations of both substrates, the observed rate constant kobs exhibited a similar Boltzmann-type decrease as observed for the apparent equilibrium constant, which could be described well by an equilibrium model expressing the observed rate constant as a function of kcat and the esterified fraction of 1 a (Figure 2D, see the Supporting Information for equations). As this decrease of kobs was further completely absent for deoxy-1 a in the presence of borate (Figures 2C and E), we concluded that borate alone does not inhibit GtPyNP, but rather the borate ester 1 a*. If GtPyNP could bind but not convert 1 a*, we reasoned that the position of the equilibrium between 1 a and 1 a* would determine the ratio of potentially active enzyme (1 a bound to GtPyNP) versus inactive enzyme (1 a* bound to GtPyNP), assuming that catalysis is a rate-limiting step. Given the highly solvent-exposed active site of pyrimidine nucleoside phosphorylases in the open state, we expected that GtPyNP should be able to accommodate the slightly twisted and sterically more demanding borate ester 1 a* and potentially allow its equilibration with the free nucleoside substrate 1 a while bound to the enzyme. Phenomenologically, such a process would resemble a classical non-competitive inhibition, consistent with our kinetic data. Indeed, MD simulations (using GROMACS61, 62 with the CHARMM3663 force field) based on our recently disclosed crystal structure of GtPyNP in complex with uridine (PDB ID 7m7k,4 see the Supporting Information for details) yielded several insights in support of the proposed model. First, an analysis of the clustered states over 50 ns simulation time indicated that the borate ester 1 a* can be bound in analogy to 1 a via hydrogen bonds with the amide motif of the nucleobase. Secondly, this analysis also showed that the average state in which the enzyme-1 a and the enzyme-1 a* complexes resided during the simulation time displayed a quite solvent-exposed active site, feasibly permitting esterification and hydrolysis processes to happen in situ. Thirdly, an examination of the distances between the domains responsible for active site closure indicated markedly reduced molecular motion of the enzyme-1 a* complex compared to the enzyme-1 a complex (Figures 2F–H and S19). While GtPyNP with bound 1 a exhibited oscillatory opening and closing motions on a timescale of around 11 ns (similar dynamics are known for the closely related thymidine phosphorylases),64-70 binding of 1 a* largely arrested this process. Specifically, binding of 1 a* locks the enzyme in an open conformation by displacing a catalytically essential arginine residue, which reversibly adopts an inward position. This appears to halt domain movement and yield an inactive enzyme, while the esterification of the 2′- and 3′-OH groups of 1 a further obstructs access to the anomeric carbon, collectively preventing productive phosphorolysis. As indicated by the clustered structures, 1 a* also slightly obstructs access to the phosphate binding site of GtPyNP. Accordingly, we experimentally observed a decreased affinity for phosphate in the presence of 1 a*, in addition to the lower kcat values stemming from pseudo-non-competitive inhibition by 1 a* (Figure 2B). Experimentally accessed and computed rate constants for the various processes involved in the catalytic cycle also proved consistent with the hypothesized mechanism. Enzyme opening/closing (ca. 0.1 ns−1) occurs on a much shorter time scale than the esterification of 1 a (ca. 3 s−1, Figure S12), catalysis (ca. 6 s−1) and substrate binding by GtPyNP (ca. 16 s−1, see the Supporting Information). Thus, the comparably slow substrate release (ca. 0.2 s−1) suggests that in situ hydrolysis of 1 a* does happen (hydrolysis pathway, Scheme 2), while we expect that the pathway comprising dissociation of 1 a* and productive binding of 1 a (dissociation pathway) is also populated significantly (see the Supporting Information for further discussions of the inhibition hypotheses). In contrast to the phosphorolysis, the kinetics in the glycosylation direction remained essentially unchanged in the presence of borate (Figures S7 and S8), providing further support for the minor role of Rib1P* in this reaction system. Consistent with the entropically driven formation of 1 a* from 1 a, we observed decreased inhibition of the phosphorolysis reaction at higher temperatures, as evident from Eyring plots obtained with different borate concentrations (Figure S9). Taken together, these results support an inhibitory mechanism phenotypically resembling a non-competitive inhibition, where rapid equilibration of 1 a to its borate ester 1 a* (both in solution and potentially while bound to the enzyme) reversibly decreases the fraction of catalytically active GtPyNP so that throughput in its catalytic cycle is primarily regulated by the position of the 1 a–1 a* equilibrium (Scheme 2). Preliminary data for other pyrimidine nucleoside phosphorylases as well as other nucleosides suggests that this inhibitory mechanism is likely not limited to GtPyNP and 1 a (Figures S1 and S18).
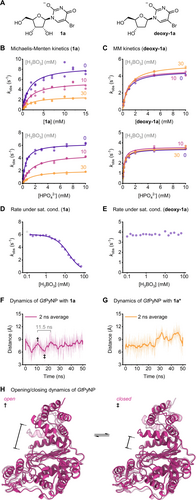
A–C Michaelis–Menten plots of the phosphorolysis of 1 a and deoxy-1 a, D and E phosphorolysis rate under saturating conditions. F–H MD simulations of GtPyNP with bound 1 a or 1 a*. See the Supporting Information for experimental details, raw data and equations.58
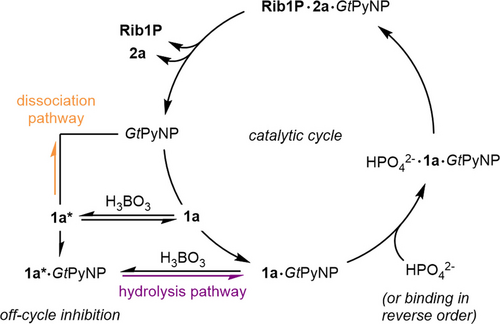
Proposed mechanism for rate decreases in the presence of borate. For simplicity's sake, the transformations of the catalytic cycle are depicted as unidirectional, although they are naturally reversible.
With a good understanding of the underlying processes governing the kinetics and thermodynamics of the phosphorolysis of 1 a in the presence of borate, we aimed to apply the observed equilibrium shifts to other nucleosides, specifically targeting glycosylation reactions. Assuming that other nucleosides would behave in analogy to our model compound 1 a, we expected that conversion shifts in glycosylation reactions would provide a general strategy to improve access to nucleosides from the precursor Rib1P (which could either be supplied as an isolated compound or generated in situ).10, 71-73 Indeed, DFT calculations for a representative set of nucleosides suggested that a variety of pyrimidine and purine nucleosides should undergo similar esterifications with borate as 1 a (Table S9), which could be confirmed by 1H NMR (ΔRG ca. −32 to −34 kJ mol−1, Figure S14) and, for two examples, with kinetic experiments (see the Supporting Information and Figure S18 for details). Although we were unable to translate the observed minor differences in Gibbs free energies to conversion shifts in transglycosylations, presumably due to a “kinetic lock” effect (see the Supporting Information and Figure S17 for details), glycosylation reactions with various pyrimidine nucleobases 2 a–f nicely reflected the expected behavior and facilitated conversion shifts of 6–17 % in favor of the respective nucleoside (Figure 3A). A similar effect could be observed for the halogenated purine 2 g when subjected to identical conditions with the promiscuous purine nucleoside phosphorylase from G. thermoglucosidasius. Lastly, an illustrative two-factor optimization for the glycosylation of 5-iodouracil (2 d) and 5-ethynyluracil (2 f, both are known for their unfavorable glycosylation thermodynamics)9, 22 showed how a balance of excess sugar donor and the “pull” effect of the corresponding nucleoside borate ester can be employed to improve the conversions in historically challenging nucleobase glycosylations (Figure 3B). For instance, when using 2 eq. of Rib1P, the conversion of 2 f to its nucleoside 1 f could be improved from 74 % to 89 % through the application of 50 mM borate, which, conventionally, would have required the application of at least 6 eq. of Rib1P. As a proof of synthetic utility, these conditions were transferred to the semi-preparative scale. The glycosylation of 10 mM 2 f with 2 eq. Rib1P proceeds under heterogeneous conditions with 42 % conversion, which improved to 77 % through the application of 50 mM borate (Figure 3C). Purification by reverse-phase HPLC cleanly yielded borate-free 1 f from this mixture in 40 % isolated yield.
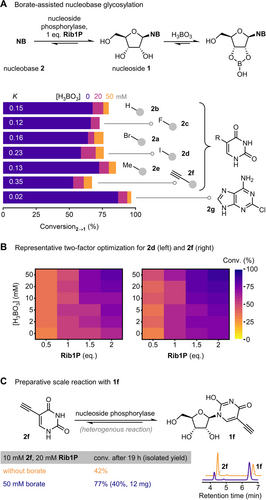
Glycosylation experiments in the presence of borate. K refers to the equilibrium constant of phosphorolysis, taken from ref [22]. In all panels, conversion refers to the conversion of the nucleobase 2 to the nucleoside 1 and derived species.
In conclusion, we characterized the equilibrium between ribosyl nucleosides and their corresponding 2′,3′-borate esters in aqueous solution, a phenomenon which facilitates apparent equilibrium shifts during nucleoside phosphorolysis and glycosylation reactions due to a biased esterification of nucleosides over the sugar phosphate Rib1P. This borate esterification also causes decreases of the phosphorolysis rate by pyrimidine nucleoside phosphorylases, most likely via non-productive binding of the nucleoside borate ester to the enzyme and its hydrolytic interconversion to the free substrate. Collectively, the effects described herein shine light on the activity of nucleoside-binding enzymes in the presence of borate and provide an additional means for reaction engineering in nucleobase glycosylation reactions. We suspect that similar processes are at play during the inhibitory processes observed for NAD+-dependent oxidoreductases and the equilibrium shifts previously reported for various enzymatic sugar epimerizations. Similarly, we anticipate that biased borate esterification could provide an avenue for engineering of a variety of different (chemo-)enzymatic reaction systems.
Acknowledgments
The authors thank the NMR department of the TU Braunschweig as well as Samantha Voges (TU Berlin) for assistance with spectroscopic experiments and Dr. M. Rhia L. Stone (Rutgers University) for comments on the manuscript. F.K. gratefully acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 492196858. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in zenodo.org at https://doi.org/10.5281/zenodo.7401178, reference number 7401178.