Biotechnological Frontiers of DNA Nanomaterials Continue to Expand: Bacterial Infection using Virus-Inspired Capsids
Graphical Abstract
Through the engineering of virus-inspired DNA-origami particles loaded with natural bacteriophage genomic cargo, scientists measured a retained functional infection in non-competent bacterial cells. Critical parameters for infectivity include structural rigidity and complete yet dynamic loading of the cargo through multivalent optimization of anchors.
Abstract
The elegant geometry of viruses has inspired bio-engineers to synthetically explore the self-assembly of polyhedral capsids employed to protect new cargo or change an enzymatic microenvironment. Recently, Yang and co-workers used DNA nanotechnology to revisit the icosahedral capsid structure of the phiX174 bacteriophage and reloaded the original viral genome as cargo into their fully synthetic architecture. Surprisingly, when using a favorable combination of structural rigidity and dynamic multivalent cargo entrapment, the synthetic particles were able to infect non-competent bacterial cells and produce the original phiX174 bacteriophage. This work presents an exciting new direction of DNA nanotech for bio-engineering applications which involve bacterial interactions.
In times where the word “virus” predominantly relates to stress and panic, scientists have used these remarkable natural structures as inspiration for the longest of times. If we (try to) forget the undeniable harm a virus can cause, we see an esthetically pleasing, geometric architecture, self-assembled from many organic building blocks, functioning in a smooth cycle of disassembly, replication and re-assembly. For many supramolecular chemists and bio-engineers alike, the structural beauty of a virus has been mesmerizing, and virus-like nanoparticles (VLNs) have been constructed from various laboratory building blocks. These bio-inspired synthetic systems predominantly borrow their physical capsid-like structure from the natural virus, and are then designed for benign applications in enzymatic catalysis or cargo protection.1 Indeed, the question of why to recreate the infective properties of a virus can be legitimately posed. To paraphrase Richard Feynman: “what we cannot make, we cannot understand”.
Whilst VLNs are often assembled from peptide or protein-based monomeric units, the quickly expanding field of DNA nanotechnology similarly demonstrates the engineering of virus-inspired geometric capsids. Examples include small icosahedral designs, with envelope virus-like lipid encapsulation2 and recently developed modular DNA triangular shells,3 which allowed the self-assembly of viral capsids ranging from 8 to an impressive 180 monomer units, and a final dimension of ≈300 nm. Paradoxically, these virus-like supramolecular structures enabled viral inactivation by trapping a natural virus in their interior, an elegant demonstration of how to use nature's own engineering against itself. Similarly, although infectivity can have controversial aspects, exploring how synthetic nanomaterials can control and simultaneously manipulate these mechanisms certainly provides interesting opportunities for biotechnological advances.
The Importance of Structural Rigidity
In the recent work of Yang and co-workers,4 not only the size and geometry of the phiX174 bacteriophage was synthetically recreated (Figure 1a, b), their DNA nano construct was subsequently loaded with the original phiX174 genome, and surprisingly showed retained infective potential in Escherichia coli (E. coli) bacterial cells. While literature on DNA-nanomaterial uptake in mammalian cells is abundant,5, 6 exploring bacterial delivery has been lagging. The authors employed a wireframe design approach,7 which allows for a relatively facile engineering of elaborate geometric architectures. However, wireframe structures are notoriously flexible, which negatively impacts a robust capsid structure. Furthermore, nanoscale rigidity is being linked to enhanced selectivity of interactions,8 a developing direction within DNA nanotechnology research and applications.

a) Structural model of the phiX174 phage, together with negative stain transmission electron microscopy (nsTEM) visualization. b) Model of the DNA based capsid-inspired icosahedron and nsTEM of the assembled structure. c) Details on the rigidification of the wireframe edges, showing the foundation layer (FL) and addition layer (AL), as well as the four-helix cross-section. Orange dots represent a free T base to reduce structural strain. Scale bar: 50 nm. Reproduced with permission.4 Copyright 2022, Wiley-VCH GmbH
To overcome flexibility hurdles, the icosahedral edges were designed on a four-helix square lattice, where two parallel helices form a foundation layer (FL, Figure 1c) and 2 parallel helices on an additional layer (AL, Figure 1c) rigidify each edge. Interestingly, experiments with more flexible architectures, lacking 10 edges, showed no bacterial infection, suggesting structural rigidity is not only key for spatially-defined functionalization but also crucial for cellular uptake, while changes in structural integrity cannot be excluded but were not reported.
The Capsid as Enthalpy Trap
As functional cargo, the original phiX174 bacteriophage genome was loaded into the DNA capsid. In the natural protein-based capsid, the long viral single-stranded DNA (ssDNA) genome is tightly packed into a tiny space during the last step of the viral life cycle. To neutralize charges, the genome coordinates with 60 copies of a positively charged protein inside the capsid. When loading the cargo into a DNA-based capsid, the repulsion of negative charges inherent to DNA causes an additional challenge. The choice of an open wireframe structure is not only interesting from a material consumption and molecular accessibility design point of view, it also limits the density of repulsive charges toward the cargo.
Specific packaging was achieved through complementary base-pairing with anchoring strands inside the capsid, in a balancing game of entropy versus enthalpy (Figure 2a). In order to force a long, flexible ssDNA molecule (high in entropy) to enter and stay inside (low entropy) the small, repulsive cavity of the DNA origami icosahedral capsid, 30 ssDNA edge-protruding handles were designed to function as directional affinity trap with the complementary region on the plasmid. Using fewer handles (e.g. 18 or 6) did not fully entrap the genome, losing the entropy battle (Figure 2b). For the entrapment to be sufficiently dynamic to allow for release, enzymatic amplification, but also for being sufficiently stable to guarantee the continued residence of the plasmid, a trade-off of 20 nucleotides (nt) length was selected as the perfect enthalpy trap, after 10 nt was found to be too dynamic and 30 nt too strong (Figure 2c). Elegantly, these handles had a dual function as primers for rolling circle amplification (RCA) of the plasmid cargo. The careful screening of affinity (length) versus valency (number) of handles to entrap the phage genome allows to understand the kinetic limits of subsequent functional assays, and to design the most adequate artificial capsid to balance cargo entrapment, uptake, and release.
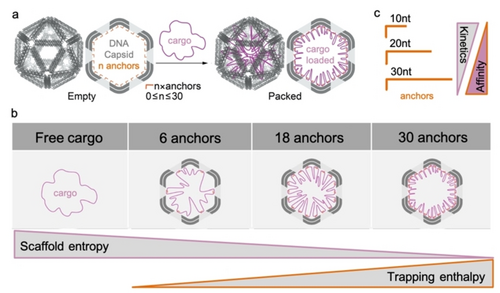
a) Packaging process of the scaffold using ssDNA anchors. b) Schematic representation of plasmid loading into capsids with increasing number of anchors (6, 18, 30). Full, yet dynamic, scaffold packing is a balance between entropic penalties in scaffold conformations and binding energy with the number of anchors. c) Increasing the anchor length improves stable trapping of the cargo, but to a cost of dynamics needed for release and transcription. Adapted with permission.4 Copyright 2022, Wiley-VCH GmbH.
Regarding uptake, the yield of this passive infection in non-competent cells was orders of magnitude weaker than the infectivity of a natural phage. Roughly, a million artificial “phages” were needed for infection, while for phiX174 almost every virus is assumed to cause infection. This stark difference suggests a diverging internalization path, which opens new potential for artificial delivery routes into bacteria, and competition with natural phage infection. Interestingly, the uptake efficiency seemed irrelevant to various types of oligolysine-PEG surface coating or addition of targeting molecules on the exterior of the artificial capsid. As the bacterial culture milieu differs from that of mammalian cells, likely other coating strategies should be investigated.
Targeting Bacteria
This study is standing at the beginning of a branch in DNA nanotechnology where applications in bacterial-centered biotechnology are found. Being able to engineer a central step in the viral life-cycle, without a priori presenting a viral particle, is innovative. While the fundamental mechanisms of how infectivity progresses are currently unknown, its functional demonstration opens several new opportunities. With bacteria being mass producers of both recombinant proteins and circular ssDNA, which served as the scaffold for DNA origami, a future development could envision the excretion of the origami-own DNA. This could be either loaded as cargo, or perhaps be a tool for the “undressing ”of the staples to release the scaffold constitutively present in the DNA origami particle, and liberate it as a template for reproduction. To control or reduce infectivity, a split plasmid approach, as recently discussed by Behler et al.,9 could be incorporated. Lastly, with bacterial delivery being a fresh target of DNA nanotechnology, many fundamental parameters including size, stability and geometry of the DNA-based architecture, which likely affect uptake yield and kinetics, are left to be explored. The doors toward bacterial targeting have been opened, and an exciting new road awaits.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.