Room-Temperature Ring-Opening Polymerization of δ-Valerolactone and ϵ-Caprolactone Caused by Uptake into Porous Pillar[5]arene Crystals
Dr. Kenichi Kato
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorKoki Maeda
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Motohiro Mizuno
Graduate School of Natural Science and Technology, Kanazawa University
NanoMaterials Research Institute (NanoMaRi), Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorProf. Dr. Yuta Nishina
Research Core for Interdisciplinary Sciences, Okayama University, Okayama, 700-8530 Japan
Search for more papers by this authorDr. Shixin Fa
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Shunsuke Ohtani
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tomoki Ogoshi
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
WPI Nano Life Science Institute, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorDr. Kenichi Kato
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorKoki Maeda
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Motohiro Mizuno
Graduate School of Natural Science and Technology, Kanazawa University
NanoMaterials Research Institute (NanoMaRi), Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorProf. Dr. Yuta Nishina
Research Core for Interdisciplinary Sciences, Okayama University, Okayama, 700-8530 Japan
Search for more papers by this authorDr. Shixin Fa
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Shunsuke Ohtani
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tomoki Ogoshi
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
WPI Nano Life Science Institute, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192 Japan
Search for more papers by this authorGraphical Abstract
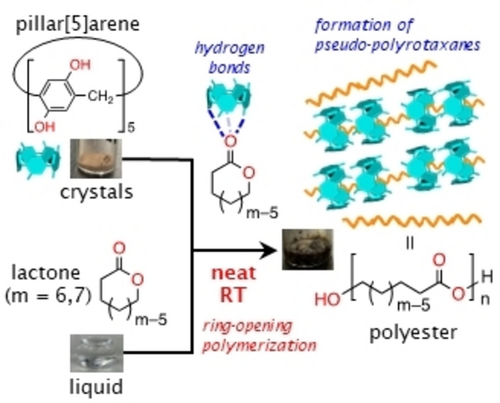
Immersion of porous phenolic pillar[5]arene crystals in liquid lactones induced ring-opening polymerization of δ-valerolactone and ϵ-caprolactone at room temperature. This is due to catalytic activity of the phenols via the hydrogen bonds and capture of linear polyesters by pillar[5]arene crystals. After the reaction, pillar[5]arene and polyesters formed pseudo-polyrotaxanes.
Abstract
Confined space provides a reaction platform with altered reaction rate and selectivity compared with a homogeneous solution. In this work, porous phenolic pillar[5]arene crystals were used as a reaction space to promote and perturb equilibrium between lactones and their corresponding polyesters. Immersion of porous pillar[5]arene crystals in liquid lactones induced ring-opening polymerization of δ-valerolactone and ϵ-caprolactone at room temperature because the phenolic hydroxy groups have catalytic activity via hydrogen bonds and the pillar[5]arene cavities prefer linear guests. After the reaction, pillar[5]arene and polyesters formed pseudo-polyrotaxanes.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202212874-sup-0001-misc_information.pdf3.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. B. Grommet, M. Feller, R. Klajn, Nat. Nanotechnol. 2020, 15, 256–271;
- 1bR. Schlögl, Angew. Chem. Int. Ed. 2015, 54, 3465–3520; Angew. Chem. 2015, 127, 3531–3589.
- 2
- 2aH. Hattori, Chem. Rev. 1995, 95, 537–558;
- 2bA. Corma, H. García, Chem. Rev. 2003, 103, 4307–4366;
- 2cF. Liu, K. Huang, A. Zheng, F.-S. Xiao, S. Dai, ACS Catal. 2018, 8, 372–391.
- 3
- 3aJ. Jiang, O. M. Yaghi, Chem. Rev. 2015, 115, 6966–6997;
- 3bL. Zhu, X.-Q. Liu, H.-L. Jiang, L.-B. Sun, Chem. Rev. 2017, 117, 8129–8176;
- 3cS. Mochizuki, T. Kitao, T. Uemura, Chem. Commun. 2018, 54, 11843–11856.
- 4
- 4aC. J. Walter, H. L. Anderson, J. K. M. Sanders, J. Chem. Soc. Chem. Commun. 1993, 458;
- 4bY. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712–717;
- 4cY. Saito, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2015, 137, 5193–5198.
- 5
- 5aK. Tanaka, F. Toda, Chem. Rev. 2000, 100, 1025–1074;
- 5bK. Biradha, R. Santra, Chem. Soc. Rev. 2013, 42, 950–967.
- 6
- 6aT. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 2008, 130, 5022–5023;
- 6bD. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang, H. Meier, Angew. Chem. Int. Ed. 2009, 48, 9721–9723; Angew. Chem. 2009, 121, 9901–9903.
- 7
- 7a Pillararene (Ed.: T. Ogoshi), The Royal Society of Chemistry, Cambridge, 2016;
- 7bP. L. Cragg, K. Sharma, Chem. Soc. Rev. 2012, 41, 597–607;
- 7cM. Xue, Y. Yang, X. Chi, Z. Zhang, F. Huang, Acc. Chem. Res. 2012, 45, 1294–1308;
- 7dN. L. Strutt, H. Huacheng, S. T. Schneebeli, J. F. Stoddart, Acc. Chem. Res. 2014, 47, 2631–2642;
- 7eT. Ogoshi, T. Yamagishi, Y. Nakamoto, Chem. Rev. 2016, 116, 7937–8002.
- 8Reviews on host–guest chemistry based on pillar[n]arenes.
- 8aT. Ogoshi, T. Yamagishi, Chem. Commun. 2014, 50, 4776–4787;
- 8bS. Ohtani, K. Kato, S. Fa, T. Ogoshi, Coord. Chem. Rev. 2022, 462, 214503;
- 8cK. Kato, S. Ohtani, S. Fa, T. Ogoshi, Bull. Chem. Soc. Jpn. 2021, 94, 2319–2328.
- 9
- 9aT. Ogoshi, Y. Nishida, T. Yamagishi, Y. Nakamoto, Macromolecules 2010, 43, 3145–3147;
- 9bT. Ogoshi, Y. Nishida, T. Yamagishi, Y. Nakamoto, Macromolecules 2010, 43, 7068–7072;
- 9cT. Ogoshi, Y. Hasegawa, T. Aoki, Y. Ishimori, S. Inagi, T. Yamagishi, Macromolecules 2011, 44, 7639–7644;
- 9dT. Ogoshi, T. Aoki, S. Ueda, Y. Tamura, T. Yamagishi, Chem. Commun. 2014, 50, 6607–6609;
- 9eJ. Chen, N. Li, Y. Gao, F. Sun, J. He, Y. Li, Soft Matter 2015, 11, 7835–7840;
- 9fK. Kato, K. Onishi, K. Maeda, M. Yagyu, S. Fa, T. Ichikawa, M. Mizuno, T. Kakuta, T. Yamagishi, T. Ogoshi, Chem. Eur. J. 2021, 27, 6435–6439;
- 9gT. Ogoshi, H. Kayama, T. Aoki, T. Yamagishi, R. Ohashi, M. Mizuno, Polym. J. 2014, 46, 77–81;
- 9hT. Ogoshi, R. Sueto, M. Yagyu, R. Kojima, T. Kakuta, T. Yamagishi, K. Doitomi, A. K. Tummanapelli, H. Hirao, Y. Sakata, S. Akine, M. Mizuno, Nat. Commun. 2019, 10, 479.
- 10
- 10aM. Labet, W. Thielemans, Chem. Soc. Rev. 2009, 38, 3484–3504;
- 10bW. Yang, K.-Q. Zhao, C. Redshaw, M. R. J. Elsegood, Dalton Trans. 2015, 44, 13133–13140;
- 10cA. Kowalski, A. Duda, S. Penczek, Macromol. Rapid Commun. 1998, 19, 567–572.
10.1002/(SICI)1521-3927(19981101)19:11<567::AID-MARC567>3.0.CO;2-T CAS Web of Science® Google Scholar
- 11B. G. G. Lohmeijer, R. C. Pratt, F. Leibfarth, J. W. Logan, D. A. Long, A. P. Dove, F. Nederberg, J. Choi, C. Wade, R. W. Waymouth, J. L. Hedrick, Macromolecules 2006, 39, 8574–8583.
- 12
- 12aY. Takashima, Y. Kawaguchi, S. Nakagawa, A. Harada, Chem. Lett. 2003, 32, 1122–1123;
- 12bY. Takashima, M. Osaki, A. Harada, J. Am. Chem. Soc. 2004, 126, 13588–13589;
- 12cM. Osaki, Y. Takashima, H. Yamaguchi, A. Harada, Macromolecules 2007, 40, 3154–3158.
- 13
- 13aT. Ogoshi, K. Kitajima, T. Aoki, S. Fujinami, T. Yamagishi, Y. Nakamoto, J. Org. Chem. 2010, 75, 3268–3273;
- 13bT. Ogoshi, D. Yamafuji, D. Kotera, T. Aoki, S. Fujinami, T. Yamagishi, J. Org. Chem. 2012, 77, 11146–11552.
- 14X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia, C. Li, Chem. Commun. 2012, 48, 2967–2969.
- 15
- 15aK. N. Houk, A. Jabbari, H. K. Hall, C. Aleman, J. Org. Chem. 2008, 73, 2674–2678;
- 15bM. K. Stedjan, J. D. Augspurger, J. Phys. Org. Chem. 2015, 28, 298–303.
- 16Strain energies were computed at the MP2/cc-PVDZ level including vibrational zero-point energies (see Supporting Information for details).
- 17L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang, Y.-W. Yang, Adv. Mater. 2014, 26, 7027–7031.
- 18Reviews on pseudo-polyrotaxanes.
- 18aF. Huang, H. W. Gibson, Prog. Polym. Sci. 2005, 30, 982–1018;
- 18bA. Harada, A. Hashidzume, H. Yamaguchi, Y. Takashima, Chem. Rev. 2009, 109, 5974–6023;
- 18cM. Arunachalam, H. W. Gibson, Prog. Polym. Sci. 2014, 39, 1043–1073;
- 18dK. Yang, S. Chao, F. Zhang, Y. Pei, Z. Pei, Chem. Commun. 2019, 55, 13198–13210;
- 18eK. Kato, S. Fa, S. Ohtani, T.-H. Shi, A. M. Brouwer, T. Ogoshi, Chem. Soc. Rev. 2022, 51, 3648–3687.
- 19
- 19aT. Ogoshi, R. Sueto, K. Yoshikoshi, Y. Sakata, S. Akine, T. Yamagishi, Angew. Chem. Int. Ed. 2015, 54, 9849–9852; Angew. Chem. 2015, 127, 9987–9990;
- 19bT. Ogoshi, R. Sueto, Y. Hamada, K. Doitomi, H. Hirao, Y. Sakata, S. Akine, T. Kakuta, T. Yamagishi, Chem. Commun. 2017, 53, 8577–8580.