Tailored Cross-β Assemblies Establish Peptide “Dominos” Structures for Anchoring Undruggable Pharmacophores
Limin Zhang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMengzhen Li
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMinxuan Wang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorLingyun Li
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMingmei Guo
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorYubin Ke
Spallation Neutron Source Science Center, Dongguan, 523803 P. R. China
Search for more papers by this authorPeng Zhou
College of Chemical Engineering, China University of Petroleum (East China), 66 Changjiang West Road, Qingdao, 266580 P. R. China
Search for more papers by this authorCorresponding Author
Weizhi Wang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorLimin Zhang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMengzhen Li
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMinxuan Wang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorLingyun Li
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorMingmei Guo
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorYubin Ke
Spallation Neutron Source Science Center, Dongguan, 523803 P. R. China
Search for more papers by this authorPeng Zhou
College of Chemical Engineering, China University of Petroleum (East China), 66 Changjiang West Road, Qingdao, 266580 P. R. China
Search for more papers by this authorCorresponding Author
Weizhi Wang
Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Institute of Engineering Medicine, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
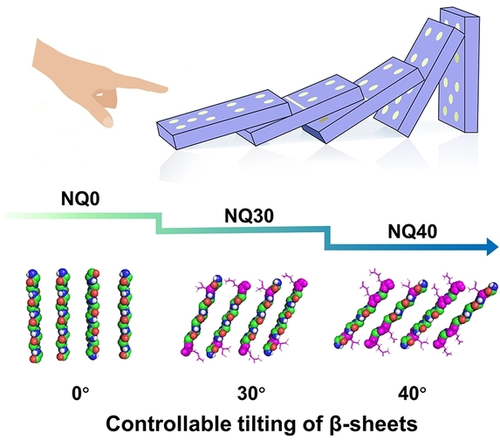
β-sheets have the ability to hierarchically stack into assemblies, and much effort has been spent on designing different peptides to regulate their assembly behaviors. Although the progress is remarkable, it remains challenging to manipulate them in a controllable way for achieving both tailored structures and specific functions. In this study, we obtained bola-like peptides using de novo design and combinatorial chemical screening. By regulating the solvent-accessible surface area of the peptide chain, a series of assemblies with different tilt angles and active sites of the β-sheet were obtained, resembling collapsed dominos. The structure-activity relationship of the optimized peptide NQ40 system was established and its ability to target the PD-L1 was demonstrated. This study successfully established the structure-function relationship of β-sheets assemblies and has positive implications on the rational design of peptide assemblies that possess recognition abilities.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202212527-sup-0001-misc_information.pdf8.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Xing, Z. Peng, W. Li, K. Wu, Acc. Chem. Res. 2019, 52, 1048–1058;
- 1bD. Pochan, O. Scherman, Chem. Rev. 2021, 121, 13699–13700;
- 1cS. Basak, I. Singh, A. Ferranco, J. Syed, H. B. Kraatz, Angew. Chem. Int. Ed. 2017, 56, 13288–13292; Angew. Chem. 2017, 129, 13473–13477.
- 2
- 2aT. M. Hermans, Nat. Nanotechnol. 2016, 11, 920–921;
- 2bG. Ghosh, R. Barman, A. Mukherjee, U. Ghosh, S. Ghosh, G. Fernandez, Angew. Chem. Int. Ed. 2022, 61, e202113403; Angew. Chem. 2022, 134, e202113403;
- 2cK. Liu, R. Xing, Q. Zou, G. Ma, H. Möhwald, X. Yan, Angew. Chem. Int. Ed. 2016, 55, 3036–3039; Angew. Chem. 2016, 128, 3088–3091.
- 3
- 3aA. Levin, E. Gazit, T. A. Hakala, L. Schnaider, T. P. J. Knowles, Nat. Chem. Rev. 2020, 4, 615–634;
- 3bY. Chen, Y. Yang, A. A. Orr, P. Makam, B. Redko, E. Haimov, Y. Wang, L. J. W. Shimon, S. Rencus-Lazar, M. Ju, P. Tamamis, H. Dong, E. Gazit, Angew. Chem. Int. Ed. 2021, 60, 17164–17170; Angew. Chem. 2021, 133, 17301–17307;
- 3cH. Wang, Z. Feng, B. Xu, Angew. Chem. Int. Ed. 2019, 58, 10423–10432; Angew. Chem. 2019, 131, 10532–10541;
- 3dJ. Li, Z. Chen, M. Zhou, J. Jing, W. Li, Y. Wang, L. Wu, L. Wang, Y. Wang, M. Lee, Angew. Chem. Int. Ed. 2016, 55, 2592–2595; Angew. Chem. 2016, 128, 2638–2641.
- 4
- 4aH. C. Fry, J. M. Garcia, M. J. Medina, U. M. Ricoy, D. J. Gosztola, M. P. Nikiforov, L. C. Palmer, S. I. Stupp, J. Am. Chem. Soc. 2012, 134, 14646–14649;
- 4bS. Mondal, E. Gazit, ChemNanoMat 2016, 2, 323–332.
- 5P. N. Cheng, J. D. Pham, J. S. Nowick, J. Am. Chem. Soc. 2013, 135, 5477–5492.
- 6
- 6aH. Cui, A. G. Cheetham, E. T. Pashuck, S. I. Stupp, J. Am. Chem. Soc. 2014, 136, 12461–12468;
- 6bW. S. Childers, A. K. Mehta, R. Ni, J. V. Taylor, D. G. Lynn, Angew. Chem. Int. Ed. 2010, 49, 4104–4107; Angew. Chem. 2010, 122, 4198–4201.
- 7Y. Zhao, L. Zhang, X. Zhou, H. Xu, X. Li, D. Wang, C. Chen, J. Wang, L. Wang, W. Wang, J. Colloid Interface Sci. 2022, 608, 1685–1695.
- 8M. Wang, J. Wang, P. Zhou, J. Deng, Y. Zhao, Y. Sun, W. Yang, D. Wang, Z. Li, X. Hu, S. M. King, S. E. Rogers, H. Cox, T. A. Waigh, J. Yang, J. R. Lu, H. Xu, Nat. Commun. 2018, 9, 5118.
- 9E. T. Pashuck, S. I. Stupp, J. Am. Chem. Soc. 2010, 132, 8819–8821.
- 10
- 10aT. J. Moyer, H. Cui, S. I. Stupp, J. Phys. Chem. B 2013, 117, 4604–4610;
- 10bE. T. Pashuck, H. Cui, S. I. Stupp, J. Am. Chem. Soc. 2010, 132, 6041–6046;
- 10cR. Tycko, K. L. Sciarretta, J. P. Orgel, S. C. Meredith, Biochemistry 2009, 48, 6072–6084;
- 10dC. M. Dobson, Nature 2005, 435, 747–748;
- 10eA. K. Mehta, K. Lu, W. S. Childers, Y. Liang, S. N. Dublin, J. Dong, J. P. Snyder, S. V. Pingali, P. Thiyagarajan, D. G. Lynn, J. Am. Chem. Soc. 2008, 130, 9829–9835.
- 11
- 11aW. J. Jeong, J. Bu, Y. Han, A. J. Drelich, A. Nair, P. Kral, S. Hong, J. Am. Chem. Soc. 2020, 142, 1832–1837;
- 11bM. Konstantinidou, T. Zarganes-Tzitzikas, K. Magiera-Mularz, T. A. Holak, A. Domling, Angew. Chem. Int. Ed. 2018, 57, 4840–4848; Angew. Chem. 2018, 130, 4932–4940.
- 12A. M. Watkins, P. S. Arora, ACS Chem. Biol. 2014, 9, 1747–1754.
- 13T. G. Barclay, K. Constantopoulos, J. Matisons, Chem. Rev. 2014, 114, 10217–10291.
- 14
- 14aY. Hu, R. Lin, P. Zhang, J. Fern, A. G. Cheetham, K. Patel, R. Schulman, C. Kan, H. Cui, ACS Nano 2016, 10, 880–888;
- 14bY. Zhao, X. Hu, L. Zhang, D. Wang, S. M. King, S. E. Rogers, J. Wang, J. R. Lu, H. Xu, J. Colloid Interface Sci. 2021, 583, 553–562;
- 14cY. Zhao, L. Deng, W. Yang, D. Wang, E. Pambou, Z. Lu, Z. Li, J. Wang, S. King, S. Rogers, H. Xu, J. Lu, Chem. Eur. J. 2016, 22, 11394–11404.
- 15G. Gao, Y. Jiang, H. Jia, W. Sun, Y. Guo, X. Yu, X. Liu, F. Wu, Biomaterials 2019, 223, 119443.
- 16Z. Álvarez, A. N. Kolberg-Edelbrock, I. R. Sasselli, J. A. Ortega, R. Qiu, Z. Syrgiannis, P. A. Mirau, F. Chen, S. M. Chin, S. Weigand, E. Kiskinis, S. I. Stupp, Science 2021, 374, 848–856.
- 17A. Aggeli, M. Bell, N. Boden, J. N. Keen, T. C. B. McLeish, I. Nyrkova, S. E. Radford, A. Semenov, J. Mater. Chem. 1997, 7, 1135–1145.
- 18J. W. Brauner, C. Dugan, R. Mendelsohn, J. Am. Chem. Soc. 2000, 122, 677–683.
- 19R. Nelson, M. R. Sawaya, M. Balbirnie, A. Ø Madsen, C. Riekel, R. Grothe, D. Eisenberg, Nature 2005, 435, 773–778.
- 20R. L. Baldwin, Protein Sci. 2000, 9, 207.
- 21J. Li, J. Wang, Y. Zhao, P. Zhou, J. Carter, Z. Li, T. A. Waigh, J. R. Lu, H. Xu, Coord. Chem. Rev. 2020, 421, 213418.
- 22
- 22aY. Zhao, J. Wang, L. Deng, P. Zhou, S. Wang, Y. Wang, H. Xu, J. R. Lu, Langmuir 2013, 29, 13457–13464;
- 22bH. Shao, J. R. Parquette, Angew. Chem. Int. Ed. 2009, 48, 2525–2528; Angew. Chem. 2009, 121, 2563–2566.
- 23M. Wang, G. Lv, H. An, N. Zhang, H. Wang, Angew. Chem. Int. Ed. 2022, 61, e202113649; Angew. Chem. 2022, 134, e202113649.
- 24H. LeVine III, Amyloid 2005, 12, 5–14.
- 25B. Zhang, R. B. Kumar, H. Dai, B. J. Feldman, Nat. Med. 2014, 20, 948–953.